Influenza Program 2018 - Presented by Angela Newbound SA PHN Immunisation Hub Coordinator - sapmea
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
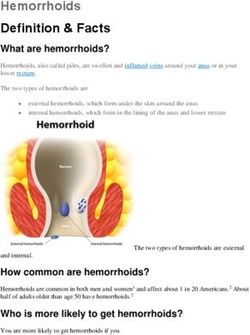
Influenza
Program 2018
Presented by Angela Newbound
SA PHN Immunisation Hub Coordinator
The Immunisation Hub thanks the Immunisation Section – CDCB for
preparing this presentationObjectives Better understanding of: • Influenza Vaccines Available for 2018 • Eligible Groups for Funded Vaccine Program • How to Order Influenza Vaccines • Vaccine Safety • Influenza Resources
Influenza virus-review
• Reservoir
• Influenza A – may infect both humans and animals
• Influenza B & C – humans the only known reservoir
• Transmission
• Direct contact with infected persons
• Contact with contaminated objects e.g. doorknobs, toys
• Inhalation of virus-laden aerosols (coughing, sneezing, and talking)
Image: Courtesy of Centres for Disease Control and PreventionIncubation Period & Infectious
Period
• Incubation period:
• Typically 3 or 4 days, range 1 to 7 days
• Infectious period:
• 24 hours before onset to 7 days after onset
• Low level of infectiousness after 5 days
• Patients no longer infectious if well AND 24 hours after resolution of fever (usually 5
to 7 days)
• Also not infectious if 24 hours after resolution of fever AND 72 hours of antiviral
medication
• Infectious for longer in children and people with weakened immune systemsInfluenza: Clinical Features
• Influenza is more than a bad cold
• Symptoms
• Chills
• Shakes
• Headache
• Muscle aches
• Fever
• Dry cough
• Respiratory complaints
• Sometimes abdominal complaints (such as pain and diarrhoea) and involvement of
other body systems occurs
• Flu is often ‘self-limiting’ but it can cause severe illness and life threatening complicationsComplications
• Complications can include
• Pneumonia
• Myocarditis
• Neurological complications
• Secondary bacterial infections, such as pneumoniaInfluenza Occurrence in Australia Annual statistics • predominantly between late Autumn and early Spring • all year round in the tropics • 5-20% of the population may be affected • 1500 to 2500 deaths • 18,000 hospitalisations • 300,000 GP consultations • $85m in health system costs
Classification of subtypes Naming is expressed in this order • Virus type • Geographic site where it was first isolated • Strain number • Year of isolation • Virus subtype
Influenza in South Australia
• 2016 there were 7,871 influenza notifications to CDCB
• 2017 saw the highest influenza notification rates in the last 10 years with
27,655 confirmed influenza notifications reported to CDCB
• 2017 season saw a 250% increase in influenza cases from the previous year
Note: The 2009 Pandemic only reported 10,763 cases on influenza in South
AustraliaNIP Influenza Vaccine Brands for
2018
Vaccine Brand 6 months to less than 3 years 3+ 18+ 65+
FluQuadri™ Junior
Fluarix® Tetra
FluQuadri™
Afluria® Quad
Fluzone® High Dose Vaccine
Fluad™ Adjuvanted Vaccine Strains Covered in 2018 QIV
vaccines
• Influenza A/ Michigan H1N1
Singapore H3N2*
• Influenza B/ Phuket
Brisbaneᵝ
*Was Hong Kong strain in 2017
ᵝThis strain is not in the trivalent vaccineNew 2018 Trivalent Influenza Vaccines Fluzone® - Sanofi Pasteur • High Dose Vaccine • Trivalent Influenza Vaccine • 0.5ml IM Injection Fluad™ - Seqirus • Adjuvanted Vaccine • Trivalent Influenza Vaccine • 0.5ml IM Injection
Fluad™ and Fluzone® • Fluad™ and Fluzone® can only be given to people ≥65 • Cannot be given to pregnant women and children • People aged 65 and older can have the QIV instead of TIV if they choose
Immunogenicity of High Dose and
Adjuvanted TIV Influenza vaccines
• Immune Systems become weaker with age which can be further compromised with
medical conditions and medical therapies
• High dose flu vaccines promote a better immune response in persons 65yrs and older
Studies* have shown that in a group that were given the high dose vaccine they showed:
• Higher Antibody responses
• Better protection against lab confirmed influenza illness
• Reduced risk of respiratory-related hospital admissions from nursing home residents
aged 65 years and older
*Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults
http://www.nejm.org/doi/full/10.1056/NEJMoa1315727?query=featured_home
Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents
admitted to hospital: a cluster-randomised trial-Lancet Respiratory Medicine
http://www.thelancet.com/journals/lanres/article/PIIS2213-2600%2817%2930235-7/fulltextGroups eligible to receive
funded vaccine
• Pregnant Women
• Aboriginal Children aged 6 months to less than 5 years
• Aboriginal persons aged 15 years or older
• Medical at risk* persons aged 6 months and older
• Persons aged 65 years and older (Trivalent or
Quadrivalent)
*For Medical at Risk groups see NCIRS Influenza fact sheetVaccine Ordering & Delivery
• Providers can commence ordering from 9th April 2018
• Deliveries will commence from 10th April 2018
• Refer to your delivery schedule for your fortnightly delivery dates and order
cut off times
• Only order the number of vaccines that can be safely stored in your fridge
without over crowding
• Providers must estimate numbers of each vaccine brand required for
different eligible groupsWeekly Ordering
• After the first fortnight, weekly orders can be requested
• Special delivery requests outside of scheduled delivery times due to running
out of stock are considered on a case by case basis
• Educate staff to plan their individual flu programs realistically in line with their
vaccine
• fridge capacity and VDC
• delivery schedulesVaccine Order Splits
• Vaccine order form will split influenza vaccine into 3 different ordering
categories
• Influenza Junior (6mths to 3 yrs and over
FluQuadri, Fluarix Tetra, Afluria Quad (from 18yrs)
• Influenza from 65yrs and over
Fluzone or Fluad
Providers will receive age appropriate available flu vaccines at time of orderOnline Immunisation Handbook
10th Edition - Recommendations
• Persons with known egg allergy can be safely vaccinated with influenza
vaccines:
*Anaphylaxis - Medical Facility
*Sensitivity - Any Setting
• Two doses are recommended for persons receiving influenza vaccine for the
first time who are:
*Immunocompromised
*Children 6 months to less than 9 yearsAIR Reporting
• AIR – Whole of Life Register
• Influenza vaccine doses are to be reported to the Australian Immunisation
Register
• Will reduce likelihood of inadvertently giving the vaccine again
Staff should check AIR before administering flu vaccine to all patients to avoid
program errorsInfluenza Education
• An influenza component is available in the Understanding Vaccines for
Adult Vaccination Requirements for Workplace Programs online learning
• https://immunisationeducation.sahealth.sa.gov.au/Influenza Resources • Posters and pamphlets can be ordered from the Commonwealth DOH website https://beta.health.gov.au/resources • 2018 Influenza Program schedules, Vaccine safety leaflets and Record of immunisation cards are available from SA Health www.poscat.com.au • Pharmaceutical companies have other promotional influenza materials available • http://au.gsk.com/ • www.sequris.com.au • www.sanofi.com.au
Commonwealth Resources
References and Useful
Websites
• Immunisation Section SA Health - Immunisation Providers
http://www.sahealth.sa.gov.au/immunisationprovider
• Australian Department of Health and Ageing
- Immunise Australia Program
th
- Immunisation Handbook 10 ed.
www.health.gov.au/immunistion
• Australian Government Department of Health
https://beta.health.gov.au/topics/immunisation
• National Centre for Immunisation Research & Surveillance (NCIRS)
http://www.ncirs.edu.au
• Immunisation Coalition
http://www.immunisationcoalition.org.au/SA PHN Immunisation Hub
SA PHN Immunisation Hub supports Angela Newbound
immunisation providers and community Immunisation Hub Coordinator (08) 8219 5900 or
through: 0421 168 367
anewbound@adelaidephn.com.au
• Clinical advice (phone, text, email) Alex Stevens
• Clinical support (planning catch- Immunisation Hub Project Officer (08) 8219 5900 or
ups, AIR issues) 0401 620 440
• Champion Nurse support astevens@adelaidephn.com.au
• Immunisation Provider Network
(IPN) Tracy Maynard
Nurse Consultant (Country SA PHN) (08) 8565 8909
• Education events (face to face and or 0400 858 142
webinars) Tmaynard@countrysaphn.com.au
• Resource development
• Attending community events Champion Immunisation Nurses (HAIMS)
(08) 8152 0363Thank you, any Questions?
You can also read