ART and Pregnancy/Breastfeeding Bournemouth 5th April 2019 - Catriona Waitt Senior Lecturer in Clinical Pharmacology, University of Liverpool ...
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
ART and Pregnancy/Breastfeeding
Bournemouth 5th April 2019
Catriona Waitt
Senior Lecturer in Clinical Pharmacology, University of Liverpool
Wellcome Postdoctoral Clinical Training Fellow
@CatrionaWaittOutline of Presentation Importance of research in pregnancy/ breastfeeding Pharmacokinetic changes in late pregnancy ARVs where PK differs in pregnancy Pharmacokinetics of drug transfer to breastfed infant ARV exposure to the breastfed infant Research gaps
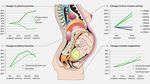
Different phases in reproductive life-cycle bring different
risk-benefit considerations
Drug-drug 1st 2nd 3rd Breastfeeding
Interactions with Trimester Trimester Trimester
Contraceptives Cells – particularly early on
Teratogenicity?
Breast milk viral load
Teratogens Dosing Dosing
Pre-conception
Infant exposure to drug –
Impact of Impact on Birth what are the effects?
maternal fetal outcomes
nausea growth Risk of late transmissions
and Impact on and infant resistance
vomiting newborn
DeliveryLong delay between licensing and pregnancy dosing data
Time to first published PK data in pregnancy
Median knowledge gap 6 years
Colbers et al. 2019, CID, epub ahead of printMomper 2018, AIDS ; 32(17):2507-2516
Elvitegravir/cobicistat pharmacokinetics in pregnant and postpartum women with HIV
Elvitegravir Cobicistat
PA EC95
C24 81% lower (T2) and 89% lower (T3) C24 60% lower (T2) and 76% lower (T3)
compared with paired postpartum compared with paired postpartumCrauwels 2019, HIV Med ; epub ahead of print
Reduced exposure to darunavir and cobicistat in HIV-1-infected pregnant women
receiving a darunavir/cobicistat-based regimen
Darunavir Cobicistat
Cmin was 92% (T2) and 89% (T3) lower Cmin was 83% (T2) and 83% (T3) lower
than in the postpartum period than in the postpartum period
Similar, clinically significant changes for unbound DRVPharmacokinetic lactation studies: FDA guidance
FDA recommendations for clinical studies in lactating
women include the following situations:
A drug under review for approval is expected to be used by women
of reproductive age
Marketed medications that are commonly used by women of
reproductive age (e.g., antidepressants, antihypertensives, anti-
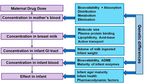
infectives, diabetic and pain medications)Maternal Drug Dose Bioavailability + Absorption
Distribution
Metabolism
Concentration in mother’s blood Elimination
Genetic differences
Molecule size
Plasma protein binding
Concentration in breast milk Lipophilicity, Acid-base
Active transport
Volume of milk ingested
Concentration in infant GI tract
Infant weight
Bioavailability, ADME
Concentration in infant blood Maturity of infant enzymes
Infant age/ maturity
Effect in infant Infant health
Pharmacodynamic factorsJ Antimicrob Chemother. 2015 Jul;70(7):1928-41
Interpretation of breast milk PK: ‘Relative Infant Dose’
Influence of pharmacogenomics
[BM] as percentage of recommended paediatric dose
TFV (given as maternal TDF): Infant concentrations not detectable
Waitt and Bonnett, CROI 2018
Abstract 55
DTG ~ 1% infant mg/Kg dose (note not recommended in infants)Questions about transmission risk: Optimisation of Regimens
• Does U=U in breastfeeding? • What are the risks of infant HIV
• What is transmission risk outside trial settings? drug resistance and toxicity?
• What is significance of cell-associated DNA? • Are any regimens safer for infant?
• What is the optimal frequency of VL monitoring? • Do any regimens have more
pharmacokinetic forgiveness?
ART in Breastfeeding:
Key Unanswered Questions
And Research Perspective
Newer Drugs Pharmacovigilance Systems
• PK of TAF and others?
• Are there subtle/ developmental risks?
• Design of lactation studies earlier
• Tiered approach to data collection (basic through to
in regulatory processes
more detailed depending on setting)
• Use of modelling to predict
• Collaboration and consensus to design data
infant drug exposure and safety
collection toolsTransmission events increasingly rare Large safety studies will require
A single centre may not get meaningful sample size international collaboration and consensus
Requires multi-centre, multi-national collaboration
Strengthened cross-disciplinary partnerships Large sample sizes and extended follow-up
Optimal design of PK studies Consensus to determine priority drugs
Use of sparse data from operational setting Surveillance tools in routine clinical care
Waitt et al, Lancet HIV 2018You can also read