Paracetamol (Acetaminophen) use in infants and children was never shown to be safe for neurodevelopment: A systematic review - Preprints.org
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
Paracetamol (Acetaminophen) use in infants and children was never
shown to be safe for neurodevelopment: A systematic review
Jasmine Cendejas-Hernandez 1, Joshua T. Sarafian 1, Victoria Lawton 1,
Antara Palkar 1, Lauren Anderson 1, Vincent Larivière 2, and William Parker 1
1
Department of Surgery, Duke University School of Medicine, Durham, NC, USA
2
École de bibliothéconomie et des sciences de l'information, Université de Montréal, Canada
Correspondence: William Parker, Ph.D.
Department of Surgery
Duke University Medical Center, Box 2605
Phone 919-681-3886
E-mail: William.Parker@Duke.edu
Running Title: Paracetamol safety and neurodevelopment
Keywords: behavior, neurodevelopment, infant, child, autism
Abstract
Although widely believed to be safe for use in infants and children when used as directed, increasing evidence
indicates that early life exposure to paracetamol (acetaminophen) may cause long-term neurodevelopmental
problems. Further, recent studies in animal models demonstrate that cognitive development is exquisitely sensitive
to paracetamol exposure during early development. In this study, evidence for the claim that paracetamol is safe was
evaluated using a systematic literature search. Publications on PubMed between 1974 and 2017 that contained the
keywords “infant” and either “paracetamol” or “acetaminophen” were considered. Of those initial 3096 papers, 218
were identified that made claims that paracetamol was safe for use with infants or children. Of these, a total of 103
papers were identified as sources of authority for the safety claim, and 36 of those contained actual experiments
designed to test safety. The 36 experiments described had a median follow-up time of 24 hours, and none monitored
neurodevelopment. Further, no trial considered total exposure to drug since birth, eliminating the possibility that the
effects of drug exposure on long-term neurodevelopment could be accurately assessed. On the other hand, abundant
and sufficient evidence was found to conclude that paracetamol does not induce acute liver damage in babies or
children when used as directed.
Page 1 of 25
© 2020 by the author(s). Distributed under a Creative Commons CC BY license.Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
Introduction
Hundreds of reports claim emphatically that, when used as directed, paracetamol (acetaminophen) is safe
for use in babies and in children. However, mounting evidence points toward the view that paracetamol exposure
during early development can have an adverse effect on neurodevelopment, even when used as directed. For
example, in a recent review [1], eight studies supporting a link between prenatal paracetamol exposure and
neurodevelopmental problems were identified [2-9]. In the three years since that review, five additional studies have
confirmed this same relationship, three of which have used data from the Norwegian Mother and Child Cohort
Study [10-14]. Although exposure to paracetamol in utero is associated with neurodevelopmental problems, even
after consideration of potentially confounding factors, the effects are typically small and the amount of paracetamol
required to yield the effect is greater than the amount typically used by average individuals. For example, after
adjusting for potential confounders such as parental education level, use of vitamin supplements, parental BMI,
smoking, and use of other drugs, Skovlund and colleagues found a weak yet significant association between prenatal
exposure to paracetamol and mother-reported communication skills: the chances of being in a lower development
category increased with increasing periods of prenatal paracetamol use but not prenatal opioid use [10]. In another
example, using propensity score matching, Vlenterie and colleagues found that 28 or more days of paracetamol use
during pregnancy was associated with a modestly increased risk of delayed motor milestone attainment (OR: 1.35,
95% CI 1.07–1.70) by children at 18 months [11].
Evidence points toward a higher risk of paracetamol-induced neurodevelopmental disorders when exposure
occurs after birth as compared to in utero. Studies using laboratory rodents demonstrate that exposure to near
therapeutic doses of paracetamol during the first days of life induces profound, long-term neurological changes [15,
16], whereas somewhat higher doses are required to induce permanent neurological damage during pregnancy [17].
These laboratory studies demonstrate that the target organ for toxicity in neonates is the central nervous system, not
the liver, and demonstrate that if paracetamol had been tested using current guidelines, it would never have been
approved for use in children. More concerning are observations in children indicating that paracetamol is not safe
for neurodevelopment. The 2008 study which first raised a red flag regarding the safety of paracetamol during
neurodevelopment found a greater than 20-fold risk of regressive autism with paracetamol use during childhood
[18]. Although this relatively small study did not attract enough interest to promote larger studies, other lines of
evidence support the view that paracetamol exposure during early life can lead to neurodevelopmental disorders. For
example, a startling 2-fold greater incidence of infantile autism in circumcised boys compared to non-circumcised
boys [19] can be readily explained by potentially negative impacts of paracetamol exposure during and following
the circumcision procedure [1]. Sadly, the widely held and entrenched belief that vaccines induce autism [20, 21]
may be yet another result of the impact of paracetamol on neurodevelopment in combination with widespread use of
the drug during vaccination [1].
With the above concerns in mind, a systematic evaluation of the peer-reviewed literature was initiated to
address the question of why paracetamol is widely believed to be safe for use during early development. All papers
published between 1974 and 2017 that contained the keywords “infant” and either “paracetamol” or
Page 2 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
“acetaminophen” were considered. All papers which made claims that paracetamol or acetaminophen is safe for use
in infants or children were identified, and the justification for this claim was critically evaluated.
Methods
As a first step in understanding why paracetamol is thought to be safe during early development, all titles and
abstracts in the PubMed® Database with keywords “infant” and “acetaminophen or paracetamol” published between
1974 and 2017 were identified. The term “infant” rather than “child” was selected because (a) the number of papers
with the term “child” was prohibitively large, and (b) the focus of the study was intended to be on drug exposure
during early development, from birth to age approximately 6 years, not individuals up to the age of 17 years. In all
cases, the terms paracetamol and acetaminophen were taken to be synonymous, and no distinctions were made.
In the second step, two coauthors (JCH and JTS) independently screened all titles and abstracts. In this step,
articles that could not be obtained in English and all articles not describing use of paracetamol in humans were
eliminated from the study. Based on titles and abstracts (if available), articles were tagged which were deemed likely
to make claims regarding the safety of acetaminophen use in infants and children between birth and age 6 years.
In the third step, two coauthors (JCH and JTS), continuing to work independently, examined full texts of all
tagged titles and abstracts. Texts were examined for the following three assertions:
(a) paracetamol use is “safe” in children or infants
(b) paracetamol is the “drug of choice” in children or infants
(c) paracetamol use is “recommended” for children or infants
In cases where the terms “drug of choice” or “recommended” were used, the context was considered. In some
cases, particularly in manuscripts expressing caution regarding the use of paracetamol, these terms were not taken to
imply safety, but rather were taken to be an indicator of the common acceptance of the drug. These articles were
excluded from the study. Based on this approach, articles were tagged that were considered to have made safely
claims regarding the use of paracetamol in infants or children younger than 6 years old.
Still working independently, two coauthors (JCH and JTS) evaluated each manuscript making a claim of safety,
determining the source of authority for the stated claim. If no literature was cited to support the claim, this was
documented. In cases where the source that was cited contained another citation, that secondary reference was
obtained and evaluated. This process continued as needed until an original source or sources describing an actual
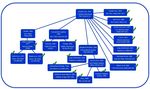
demonstration of safety was identified. An example of the results of this process is shown in Figure 1.
Page 3 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
Figure 1. Flow diagram illustrating connections between articles claiming that paracetamol use is safe for infants or
children when used as directed. In this example, the citations in a paper by Temple and colleagues in 2017 [25] are
assessed. Articles describing new experiments designed to test safety of paracetamol or which contain claims of
safety without citation are included in Table 2 and are indicated by a check mark. Articles shown in the diagram
which do not describe experiments designed to test safety of paracetamol and which cite other articles as a source
for claims of safety [23, 26, 27, 134] are not included in Table 2 and are not indicted by a check in the diagram.
In the fourth step, any discrepancies between the analyses provided by coauthors JCH and JTS were arbitrated
by coauthor WP. In the fifth step, articles upon which safety claims were based were compiled. Finally, articles
upon which safety claims were based and which contained experiments designed to assess safety were evaluated.
For each experiment described, the study group, endpoints measured, and follow-up time were evaluated.
Results
An overview of results from a systematic search for studies demonstrating safety of paracetamol use in infants
and children is shown in Table 1. The initial Medline search provided 3096 articles that contained the terms infant
and either paracetamol or acetaminophen that were published between 1974 and 2017. From these articles, 467 were
selected for assessment based on likelihood of safety claims regarding use of paracetamol in infants or children. Of
these 467 articles, 218 made safety claims regarding the use of paracetamol in infants or children. During this phase
of the study, numerous articles were identified which either claimed or demonstrated that paracetamol use, even at
doses beyond the recommended dose, does not generally cause long-term liver damage in infants or children. Any
claims of safety for liver function were not evaluated in detail and were not considered in this study. Only general
claims of safety were assessed.
Page 4 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
Table 1. Number of citations identified in the systematic search during each step of the study. Numbers are provided
for both analysts performing the work (JCH and JTS). The overlap is the number of citations that were the same
between the two analysts. *Numbers in parentheses indicated the number of citations in which both analysts
identified the same citation, but not the same source or sources as the authority for claims of safety.
Step JCH Overlap JTS Total
1. Medline (APAP + infant) 3096
2. Safety claim, first step 310 193 350 467
3. Safety claim, second step 189 144 (53*) 189 234
4. Safety claim, final 173 128 (37*) 173 218
5. Sources attributed to safety claim 103
6. Sources with experiments attributed to safety claim 36
Of the 218 articles making claims that paracetamol use in infants or children is safe, half (114) provided no
citation. The other half (114) of the articles cited additional articles as evidence that paracetamol is safe in infants or
children. Cited articles were carefully evaluated as described in the methods. In some cases, those “primary” cited
articles did not make original claims of safety, but rather cited additional (“secondary”) articles. In cases where a
primary article cited another article, the primary article was not considered to have made an original claim of safety,
and was not evaluated further. An example of the results of this process is shown in Figure 1. Both primary articles
and secondary (and tertiary, etc) articles attributed with claims of the safety of paracetamol use in infants or children
were compiled and are shown in Table 2. In total, 103 articles were identified which were cited as containing
original claims that paracetamol use in infants or children is safe when used as directed.
Table 2. Sources cited as stating that acetaminophen is safe for infants or children when used as directed. *This
article is cited as “Renn E. The antipyretic use of paracetamol versus ibuprofen in a pediatric care setting.
Physical Therapy. 2000;25:395-397.” This reference does not apparently exist: The volume number
corresponding to the year 2000 for the journal Physical Therapy is 80, not 25. We were unable to determine
what actual article it may have originally referred to. **The Canadian Pediatric Society paper from 1998 was
mis-cited as being from 2000 in one instance. *** This article, cited as Kehlet and Werner (2003) from the
journal Drugs, Volume 63, pp 15-22 (Spec no 2), does not exist on the journal’s website for unknown reasons.
Outcome
Sources cited for safety of paracetamol Study subjects measures related Duration of Times
in children or infants: study number, to safety or safety monitoring cited
year, and design claims made.
1. 1999: Double-blind clinical trial with 9,127 children “Serious adverse 4 weeks 13
three treatments, one of paracetamol and treated with clinical events”
two different concentrations of ibuprofen paracetamol; requiring
given for fever [22] median age is hospitalization:
14 months gastrointestinal
bleeding, renal
failure,
anaphylaxis,
Reye’s syndrome,
asthma,
bronchiolitis, and
vomiting/gastritis
Page 5 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
2. 1997: Double-blind, randomized, three- 80 children were Monitoring by 2 hours: all 9
way crossover study with three treatments, treated with parents for patients
one each of paracetamol, ibuprofen, and paracetamol, “adverse events”: received
placebo given by parents for headache. age range nausea, vomiting, paracetamol at
Each child with three migraine attacks was approximately 4 and gastric pain some point, so
treated in random order with single oral to 16 years long-term
doses of the study drugs [34] monitoring was
not feasible.
3. 1978: Editorial describing current NA: Editorial Claims: NA: Editorial 8
practice with analgesic use in children “anticipated liver
[35] damage is not
observed” based
on personal
experience and
interactions with
other clinicians
4. 2001: Review describing analgesic use NA: Review Claims: “40-year NA: Review 8
in children [36] safety record in
children” without
citation
5. 1978: Review describing antipyretic NA: Review Claims: “relatively NA: Review 7
therapy in febrile children [37] free of adverse
reactions” without
citation and 9
citations provided
for the statement
that hepatotoxicity
from paracetamol
in children is "very
low compared with
that seen in adults"
6. 2000: Renn, 2000 NA: Erroneous NA: Erroneous NA: Erroneous 7
Erroneous citation* citation citation citation
7. 2011: Report describing current NA: Report Claims: “generally NA: Report 7
recommended practice [38] regarded as safe”
without citation.
Lesko (1999) is
cited for equivalent
safety between
ibuprofen and
paracetamol.
8. 1983: Review describing pediatric NA: Review Claims: “one of the NA: Review 6
dosing of paracetamol [39] safest” without
citation
9. 1995: Double-blind clinical trial with 28,130 children Serious events 4 weeks 6
three treatments, one of paracetamol and treated with defined as
two of different concentrations ibuprofen paracetamol; hospitalization for
given for fever [40] median age is acute
40 months gastrointestinal
bleeding, acute
renal failure, or
anaphylaxis.
10. 1998: Practice guidelines** [41] NA: Practice Claims: “As NA: Practice 5
guidelines demonstrated by guidelines
the numerous
Page 6 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
prospective clinical
studies”,
paracetamol is
“remarkably safe
in therapeutic
doses”, without
citation.
11. 1972: Double-blind study with two 39 children Unspecified 6 hours 5
treatments, one each of aspirin and treated with “complications or
paracetamol, given for antipyretic effect paracetamol, side effects”
[29] age 6 months to
6 years
12. 1977: Clinical guidelines for use, NA: Clinical Considered to have NA: Clinical 4
predominantly focused on aspirin, but also guidelines a “wide range of guidelines
including paracetamol. [28] safety” based on
“the large doses of
paracetamol
required to evoke
toxic reactions” in
laboratory animals.
In addition,
considered “safe
and effective when
used as directed”,
with two studies
cited [29, 30]
13. 1978: Commentary on paracetamol NA: Claims: “safe and NA: 4
use [42] Commentary effective analgesic Commentary
and antipyretic in
usual therapeutic
dosage” without
citation.
14. 1978: Review comparing aspirin’s and NA: Review Claims: “the NA: Review 4
paracetamol’s antipyretic and analgesic choice of agents
activity [43] for antipyresis in
clinical practice
has been narrowed
to aspirin and
paracetamol”
without citation.
15. 1996: Review of paracetamol liver NA: Review Makes no general NA: Review 4
toxicity in children under the age of 6 safety claim,
years [44] although extensive
references are
provided showing
that paracetamol
does not cause
long-term damages
to infants’ livers.
16. 1997: Double-blind clinical trial with 97 children Renal function as 4 weeks 4
three treatments, one of paracetamol and treated with indicated by blood
two different concentrations of ibuprofen paracetamol; urea nitrogen
given for fever [45] median age is (BUN) and
29 months creatinine levels.
Page 7 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
17. 1972: Clinical study with three 80 children No outcome 6 hours or less 4
treatments, one each of aspirin, treated with measures
paracetamol, and a combination of the two paracetamol, specified.
given for fever [30] age 6 months to
5 years
18. 2005: Pharmacokinetic study. NA: No safety NA: 3
[46] Pharmacokinetic outcomes reported. Pharmacokineti
study No safety claims c study
made.
19. 2011: Randomized open-label study 75 patients total Changes in liver 48 hours 3
with two dosing regimens of intravenous were treated enzymes, changes
paracetamol given for analgesic or with in vital signs, and
antipyretic effect. paracetamol, 3 reported or
[47] Intravenous paracetamol contains neonates, 25 observed adverse
cysteine, an antidote for paracetamol infants, 25 drug effects, which
poisoning. The antidote is not present in children, and 22 included the
the oral medication. adolescents following: anemia,
constipation,
nausea, vomiting,
face edema,
pyrexia,
hypokalemia,
hypomagnesemia,
hypophosphatemia,
agitation,
atelectasis, pleural
effusion,
pulmonary edema,
stridor, wheezing,
periorbital edema,
and pruritus
20. 1973: Review describing precautions NA: Review Makes no safety NA: Review 2
with paracetamol use claim with respect
[48] to pediatric use.
21. 1981: Review comparing efficacy of NA: Review Claims: a “high NA: Review 2
aspirin and paracetamol in fever reduction degree of safety” at
in children therapeutic doses
[49] without citation.
22. 1992: Pharmacokinetic study in adults NA: NA: study in adults NA: 2
aged 21-25 years [50] Pharmacokinetic Pharmacokineti
study c study
23. 1993: Review [51] NA: Review Claims: “Recent NA: Review 2
data have
supported the
relative safety (and
efficiency) of
paracetamol in
newborn infants”
without citation.
24. 1996: Double-blind study with two 47 children were Extensive 36 hours 2
treatments, one of each ibuprofen and treated with assessment of
paracetamol, given for fever [52] paracetamol, adverse events.
age 0.2 to 9.4 Claims: “majority
years; median of adverse events
age is 1.6 years had a doubtful or
Page 8 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
no relationship the
treatment, and
most were
considered mild.”
25. 1997: Computer simulation used to NA: Computer No safety claims NA: Computer 2
predict dosing needed to achieve desired simulation made. simulation
concentration of drug in plasma [53]
26. 1997: Pharmacokinetic study. NA: No safety NA: 2
[54] Pharmacokinetic outcomes reported. Pharmacokineti
study. Claims: c study.
“Commonly used
in children because
of its (efficacy
and) safety”
without citation.
27. 1999: Pharmacokinetic study with a 28 preterm No safety Up to 12 hours 2
single rectal dose of paracetamol [55] neonates, 2 days outcomes stated.
after birth Claimed: “safe”
28. 2007: Comparison of efficacy between 150 patients Monitoring for 3 days 2
paracetamol and ibuprofen. First phase treated with “adverse events,”
was a single dose, double-blind paracetamol, three of which
administered in the clinic, followed by an age range were infections,
open-label phase administered at home for approximately gastrointestinal
the second and subsequent doses. [56] 0.40 to 11 years; disorders, and
average age is respiratory
3.71 years disorders.
29. 2008: Retrospective study using data 149 neonates Hepatic enzyme 48 hours 2
collected in neonates treated with total, median profiles including
intravenous paracetamol. postmenstrual ALT, AST, and
[57] age 38 weeks GGT
and median
postnatal age is
5 days
30. 2011: Review describing NSAIDs and NA: Review Makes no safety NA: Review 2
paracetamol and their roles in reducing claim.
side-effects after surgery [58]
31. 2013: Review describing dosing and NA: Review Claims: “dosing NA: Review 2
antipyretic efficacy of paracetamol [59] range is well
tolerated in
children” without
citation.
32. 1965. Pharmacology reference book NA: Review Pediatric dose NA: Review 1
[60] stated without
citation, and
without further
discussion of
pediatric use.
Makes no safety
claim.
33. 1967: Clinical comparison of a single 50 infants Unspecified 6 hours 1
dose of paracetamol, aspirin, and treated with “undesirable
salicylamide [61] paracetamol, up effects” not
to 48 months observed
old
Page 9 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
34. 1978: Review describing the NA: Review Does not discuss NA: Review 1
pathophysiology of aspirin overdosage paracetamol.
[62] Makes no safety
claims
35: 1982. Monitoring of drug use by the 1,158 children, Adverse events not NA: No follow- 1
Pediatric Drug Surveillance Program [63] up to age 16, reported. Makes no up conducted
received safety claim
paracetamol
36. 1982: An abstract [64] cited by Ragg, Not determined Not determined Not determined 1
1997 [65]
37. 1984: Prospective study observing 132 children, No side effects During inpatient 1
adverse drug reactions in pediatric age not observed. No stay: time not
inpatients. [66] specified, safety claims specified.
received an made.
antipyretic or
analgesics while
hospitalized
(paracetamol
not mentioned)
38. 1989: Editorial comparing ibuprofen NA: Editorial Claims: NA: Editorial 1
and paracetamol [67] “therapeutic doses
of either drug
[ibuprofen and
paracetamol] cause
no discernable
adverse effects”
without citation.
39. 1989: Review assessing pain in NA: Review Claims: “Recent NA: Review 1
neonates and the approaches to data have
postoperative analgesia supported the
[68] relative safety and
analgesic efficacy
of paracetamol in
newborn infants”
without citation.
40. 1990: Pharmacology reference book NA: Review Claims: “usually NA: Review 1
[69] well tolerated”
without citation,
but use in pediatric
populations is not
discussed.
41. 1991: Review describing paracetamol NA: Review Makes no safety NA: Review 1
hepatotoxicity and poisoning in children claim
[70]
42. 1992: Review describing the NA: Review Claims: NA: Review 1
hepatotoxicity of non-steroidal anti- Paracetamol is
inflammatory drugs [71] “normally very
safe when used
properly” although
this statement does
not necessarily
refer to pediatric
use
43. 1992: Randomized, double-blind, 16 children Adverse events 24 to 48 hours 1
multidose, parallel-group, variable treated with included headache,
Page 10 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
duration clinical trial with three different paracetamol; gastrointestinal
concentrations of ibuprofen and one of average age is effects, sweating,
paracetamol given for pediatric fever [72] 5.2 years hypothermia,
abdominal pain,
agitation,
nervousness, and
adverse
experiences related
to the respiratory
system.
44. 1994: Textbook [73] cited by Wilson, NA: Textbook Not determined NA: Textbook 1
1995. [74]
45. 1996: Double-blind study with two 100 children Liver enzymes 24 hours 1
treatments, paracetamol and placebo given were treated determined by
for postoperative pain [75] with blood samples.
paracetamol,
age 3 to 14
years
46. 1996: Randomized study with two 28 children, age Outcome measures 240 minutes 1
treatment groups, one of which received 2 to 8 years included pain
paracetamol preoperatively and the other scores and the need
postoperatively [76] for rescue
analgesics
47. 1997: Double-blind, multicenter study 56 children Changes in 6 hours 1
with two treatments, one each of ibuprofen treated with temperature. Only
and paracetamol, given for fever [77] paracetamol, side effect reported
age 8 months to was vomiting.
11 years;
average age is
4.2 years
48. 1999: Randomized, double-blind, 90 children Postoperative pain 24 hours 1
placebo-controlled study with four treated with was evaluated by
different concentrations of paracetamol paracetamol, behavioral
given after induction of anesthesia [78] age 1 to 7 years assessment and
physiologic
measurement.
Only side effects
reported were
postoperative
nausea and
vomiting
49. 1999: Clinical trial examining the 10 infants, up to Adverse events not First 2 days 1
efficacy and pharmacokinetics of the age of 2 reported. Claims: after birth
paracetamol in term infants (multiple- days “paracetamol can
dose) [79] be administered
safely to neonates
on the first day of
life”
50. 2000: Review describing non-opioid NA: Review Claims: rectal NA: Review 1
drugs for treatment of postoperative pain paracetamol
[80] “seems safe in
children” without
citation.
Page 11 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
51. 2000: Pharmacokinetic study of 21 children, age No sign of adverse Variable, from 1 1
postoperative, repeated dosing of rectal 9 weeks to 11 effects observed. to 5 days
paracetamol [81] years Claims:
Paracetamol has
gained wide
acceptance as a
simple and safe
antipyretic and
analgesic in
children”, without
citation.
52. 2000: Observational study of calls to a 1,019 children Parent’s report of 72 hours 1
poison center to evaluate pediatric up to the age of signs of
paracetamol exposures [82] 7 years hepatotoxicity.
53. 2000: Review of paracetamol’s NA: Review Claims: NA: Review 1
history, present and future [83] paracetamol is an
“effective and
remarkably safe
drug when used
properly” without
citation
54. 2000: Randomized, double-blind study 24 children Outcome measures 3 days 1
with two treatments, one each of treated with used were pain
diclofenac and paracetamol for paracetamol, scores and relief of
postoperative analgesia [84] age 5 to 15 pain or dysphagia.
years; median Only side effects
age is 10 years reported were
nausea and
vomiting.
55. 2000: Integrated Management of NA: Review Makes no safety NA: Review 1
Childhood Illness handbook by the World claims
Health Organization [85]
56. 2000: Randomized, double-blind, 120 patients, Disorientation, 8 to 10 hours 1
multicenter study comparing paracetamol age 2 to 11 extreme irritability,
controlled-release sprinkles and years and confusion
paracetamol immediate-release elixir in were the only
febrile children [86] adverse events
recorded.
57. 2000: Guide to pediatric medication NA: Review Claims: “Usually NA: Review 1
and nutrition [87] well tolerated
when used as
directed”, without
citation.
58. 2001: Review describing treatment NA: Review Claims: “generally NA: Review 1
with paracetamol in infants [88] considered a safe
drug” without
citation but warns
of potential
toxicity with
glutathione
depletion.
59. 2001Literature review describing NA: Review Claims: NA: Review 1
perioperative use of high-dose of rectal “administration of
paracetamol [89] high-dose rectal
paracetamol in the
Page 12 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
perioperative
period appears to
be safe” without
citation.
60. 2001: Review describing paracetamol NA: Review Claims: safety NA: Review 1
toxicity in children [90] based on NAPQI
production and
glutathione levels
without citation.
61. 2001: Review describing the NA: Review Makes no safety NA: Review 1
neurobiology of pain [91] claim
62. 2001: Randomized, stratified, placebo- NA: minimum NA: minimum age NA: minimum 1
controlled, single-dose, double-blind, age 16 years, 16 years, average age 16 years,
triple-dummy, single-center, parallel- average age age 22.2 years average age
group study with four treatments, one each 22.2 years 22.2 years
of ibuprofen, ketoprofen, paracetamol, and
placebo given for postoperative dental
pain [92]
63. 2001: Blinded study conducted to 40 patients, Paracetamol 24 hours 1
observe the analgesic efficacy of rectal average age is plasma
and oral paracetamol in two separate 10.3 years concentrations and
groups in children after craniofacial pain scores. Only
surgery side effect reported
[93] was vomiting.
Makes no safety
claims.
64. 2002: Review comparing the effects of NA: Review Claims: “low NA: Review 1
paracetamol, NSAIDs or their incidence of
combination in postoperative pain adverse effects”
management [94] without citation
65. 2002: Literature review describing NA: Review Claims: “Both NA: Review 1
paracetamol and ibuprofen use for fever drugs appeared
treatment in children [95] well tolerated and
no evidence of
difference in short-
term adverse
effects was
observed” without
citation
66. 2003: Editorial describing use of NA: Editorial Claims: NA: Editorial 1
antipyretics [96] paracetamol is
“traditional(ly)
considered to be
safe based on (a)
large clinical
experience over (a)
long time” without
citation.
67. 2003: Erroneous or out of print NA: Erroneous NA: Erroneous or NA: Erroneous 1
citation*** [97] or out of print out of print citation or out of print
citation citation
68. 2003: Review describing anti- NA: Review Claims: NA: Review 1
inflammatory agents and paracetamol in “paracetamol
neonates [98] remains the drug of
choice for
Page 13 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
antipyresis in
neonates” and “the
adverse effect of
paracetamol is
more favorable”
without citation
69. 2003: Randomized, double-blind, 80 children Adverse events All children 1
placebo-controlled study with four treated with defined as were kept in the
treatments groups, ibuprofen, paracetamol, paracetamol, retching, vomiting, PACU for 1.5
a combination of the two, and placebo. age 1 to 6 years; abdominal pain, hours. The
The purpose was to observe the analgesic average age is and dizziness parents of the
efficacy of each treatment [99] 2.7 years children were
asked to record
the well-being
of their child
until 24 hours
after anesthesia.
70. 2004: Pharmacokinetic study with a 30 neonates, 24 Liver enzymes 10 hours 1
single intravenous dose of propacetamol hours after birth determined by
[100] blood samples.
71. 2004: Systematic review assessing the NA: Review Makes no safety NA: Review 1
prevalence of aspirin induced asthma in claim with respect
adults and children and other issues to pediatric use.
related to the syndrome [101]
72. 2005: Review describing NA: Review Claims: NA: Review 1
paracetamol’s tolerability profile [102] “Paracetamol is a
very well tolerated
drug at therapeutic
doses” without
citation, although
this statement does
not necessarily
refer to pediatric
use.
73. 2005: Randomized, double-blind study 25 children Agitation in 24 hours 1
with three treatments, one each of treated with recovery measured
ibuprofen, paracetamol, and placebo given paracetamol, using Oucher’s
before surgery [103] age 3 to 12 scale
years
74. 2005: Evaluation of pain management 37 children, age Evaluation of 16 to 20 hours 1
guidelines for tonsillectomy [104] 5-11 years. nausea and
vomiting.
75. 2006: Practice guidelines [105] NA: Practice Makes no safety NA: Practice 1
guidelines claim guidelines
76. 2006: Practice guideline to assist NA: Guidelines Makes no safety NA: Guidelines 1
poison center personnel with management claim
of paracetamol poisoning [106]
77-79. 2004-2010: Three textbooks [107- NA: Textbooks Not determined NA: Textbooks 1
109] cited by Karbasi and colleagues
[110]
80. 2007: Review describing paracetamol NA: Review Claims: “an NA: Review 1
safety and hepatotoxicity [111] excellent overall
safety record” with
infants and
Page 14 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
children without
citation
81. 2007. Open-label, single-sequence, NA: study in NA: study in adults NA: study in 1
multiple-dose study with intravenous adults adults
paracetamol in adults [112]
82. 2007: Randomized double-blind 103 children Outcome measures 6 hours 1
placebo-controlled study with paracetamol treated with included fever
given for fever [113] paracetamol, clearance time, rate
age 6 months to of fall of
6 years; average temperature,
age is 26.1 percent reduction
months of temperature,
proportion of
afebrile children,
symptomatic
improvement and
clinical and
biochemical
adverse effects.
Claims:
“considered to be a
safe drug at
therapeutic levels.”
83. 2007: Randomized, double-blind, 30 children Need for Minimum of 2 1
placebo-controlled study with three treated with postoperative hours
treatments, one each of naproxen, paracetamol, rescue fentanyl and
paracetamol, and placebo before the age 1 to 6 years, the incidence of
induction of anethesia [114] average age is postoperative
1.3 years nausea and
vomiting.
84. 2007: Study with zolmitriptan nasal NA: study not NA: study not NA: study not 1
spray, not paracetamol [115] involving involving involving
paracetamol paracetamol paracetamol
85. 2007: Guidelines for assessment and NA: Clinical Makes no safety NA: Clinical 1
initial management of fever in children guidelines claim guidelines
younger than 5 years [116]
86. 2007: Review describing systemic NA: Review Claims: “when the NA: Review 1
analgesics for children [117] maximum daily
dose of
paracetamol is
observed it is well
tolerated” without
citation
87. 2009: Comparative study with three 112 children Children were Up to 48 hours 1
treatments: paracetamol, ketoprofen, and were treated monitored without
ibuprofen given for fever [118] with observation of
paracetamol, drug-related side
average age effects. Makes no
about 4 years safety claim
old.
88. Randomized, controlled trial in which 29 infants were Did not report any 48 hours 1
patients received either paracetamol or treated with adverse events.
placebo for postoperative pain [119] paracetamol, Measured the
age 0-2 months efficacy of
Page 15 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
paracetamol, not
safety.
89. 2009: Review describing the Italian NA: Review Claims: NA: Review 1
Pediatric Society guidelines on the paracetamol is
management of fever in children [120] “generally well
tolerated” without
citation.
90. 2009: Review describing drugs of NA: Review Makes no safety NA: Review 1
choice for sedation and analgesia in the claims
NICU [121]
91. 2009: Review describing the peri- NA: Review Claims: NA: Review 1
operative use of paracetamol [122] paracetamol is a
“safe, well-
tolerated drug with
proven efficacy”
without citation.
92. 2009: Systematic review of the clinical NA: Review Makes no safety NA: Review 1
safety and tolerability of ibuprofen claims
compared with paracetamol in pediatric
pain and fever [123]
93. 2009: Online survey of anesthetists NA: Survey Makes no safety NA: Survey 1
and the current prescribing practice of i.v. claims
paracetamol [124]
94: 2010: Hemodynamic study with 72 neonates, age Assessment of 6 hours 1
intravenous paracetamol in neonates [125] 1 to 27 days; hemodynamics. No
average age is 3 safety claims
days made.
95: 2010: Review describing post- NA: Review Makes no safety NA: Review 1
operative pain management [126] claim
96: 2010: Meta-analysis of efficacy and NA: Review Claims similar NA: Review 1
safety of ibuprofen and paracetamol in safety profiles
children and adults [127] between
paracetamol and
ibuprofen, but
makes no absolute
safety claim
97: 2011: Study of efficacy and safety in NA: study in NA: study in adults NA: study in 1
adults [128] adults adults
98: 2011: Literature review of clinical NA: Review Claims: it “has NA: Review 1
trials of intravenous paracetamol for been well known
postoperative pain [129] as a safe and
effective” without
citation
99: 2012: Review of efficacy and NA: Review Makes no safety NA: Review 1
pharmacokinetics of paracetamol in claims
pediatric patients [130]
100: 2012: Retrospective study using data 230 patients, Postoperative fever 1 year 1
collected on pediatric surgery patients to age 0 to 15 and its etiologies,
identify the status and risk factors of major years, average mortality
infections [24] age is 4.28 years discharge, and
rates of re-open
sternotomy
reintubation
Page 16 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
101. 2013: Case series evaluating the 10 preterm Pre- and 3 days 1
efficacy of intravenous paracetamol in infants, age 2 to posttreatment
preterm infants with hemodynamically 15 days levels of liver
significant patent ductus arteriosus enzymes
(hsPDA) [131]
102. 2013: Mechanistic study in NA: Study in Makes no safety NA: Study in 1
laboratory animals [132] laboratory claim laboratory
animals animals
103. 2014. Literature review assessing NA: Review Claims: “doses of NA: Review 1
liver toxicity due to paracetamol in less than 75
children [133] mg/kg/day of
paracetamol are
safe for children
younger than 6
years of age”
without citation
Several studies emerged as popular citations for the claim that paracetamol use in infants or children is safe
when used as directed. Only 19 articles were cited more than twice, and the most popular article [22] was cited a
total of 13 times (Table 2) by the 218 articles we identified. However, in some cases, well cited articles did not make
original claims of safety, and are therefore not included in Table 2. For example, an article by Perrott and colleagues
in 2004 [23] was cited a total of 7 total times by the 218 articles we identified. However, Perrott’s article, being a
review, does not make original claims of safety, but rather cites additional articles as the authority for assurance of
safety (Figure 1). Thus, Perrott’s article is not included in Table 2 as an original source for the claim that use of
paracetamol is safe for infants and children when used as directed.
Of the 103 articles cited as authority for the safety of paracetamol use in infants or children, 27 did not make
claims of safety and did not address safety experimentally (Table 2). Thus, 76 of the 103 articles did address safety,
and 48 of these (63%) had already been identified in the original 218 articles culled from the Medline search. Of the
103 articles, 36 articles described experimental studies which involved paracetamol use in infants or children.
Although several of these studies provided measures of liver function (Table 2), none of these 36 studies provided
any assessment of neuropsychiatric function. Further, the median follow-up time of all 36 studies was 24 hours
(Figure 2), far too short to identify any long-term effects of drug exposure on neuropsychiatric function. Four
studies had a follow-up time of longer than 10 days, with one study in particular [24] evaluating patients out to one
year. However, these studies were blind to any potential effects of drug exposure on long-term neuropsychiatric
function. For example, although patients were followed for a full year in one study [24], the only endpoint measured
was re-admission for surgery.
Page 17 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
Figure 2. Maximum follow-up times for 34 of the 36 studies describing experiments designed to test the safety
of acetaminophen in infants or children. One study [24] monitoring readmission for surgery for one year is omitted
from the graph. Another study [66] observing patients during their inpatient visit did not specify duration of
monitoring, and therefore could not be included in the graph. The three studies monitoring outcomes for 4 weeks
(shown on the graph) did not monitor neuropsychiatric function.
The path from more recent papers to the original research addressing the safety of paracetamol in infants
and children was sometimes convoluted. In one notable case, a popular citation did not did appear the literature
(Table 2). Not only did the volume and journal number not match, but the title could not be found elsewhere. As
another example, the citations reporting safety of paracetamol use in children reported by Temple and colleagues in
2017 [25] are illustrated in Figure 1. This article provides a detailed description of three prior reports to the
European Medicines Agency (reports 24570, 24571, and 47402) which, together, according to the authors, “confirm
that the recommended standard paracetamol dose of 10 to 15 mg/kg is a safe and effective dose for use in pediatric
patients when administered as a single dose or as multiple doses for up to 72 hours.” However, the only safety
measure used in the three studies was ALT levels as a marker for liver function, assessed for a maximum of 72
hours. In addition to the three reports described in their publication, Temple and colleagues cite 10 additional
articles as sources for safety, including the claim that paracetamol has a “well-established efficacy and favorable
safety profile” (Figure 1). Among these 10 papers is a clinical trial [26] that addresses efficacy but not safety, and
refers to two other papers that address safety, one by Lesko [22]. The paper by Lesko contradicts the view that
paracetamol is safe, finding that paracetamol is significantly worse than ibuprofen in terms of risk for outpatient
visits following treatment of children with asthma. Another of the articles cited by Temple in 2017, a review written
by Temple more than 30 years before [27], cites a paper in the Federal Register [28] as the source for the statement
Page 18 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
that “Paracetamol is relatively free of side effects and has a wide margin of safety between therapeutic doses and
toxic doses.” The document in the Federal Register [28], a lengthy treatise primarily focused on determination of the
appropriate dose for adults of salicylates in general and aspirin in particular, in turn cites two papers involving safety
studies of paracetamol in the human pediatric population. One of those studies [29] evaluated 98 children using a
blinded approach comparing aspirin and paracetamol, and monitored the children for only 6 hours. The other study
[30] monitored 20 children following administration of both aspirin and paracetamol. In that study, monitoring
occurred for 6 hours or less and no information was provided regarding particular side effects that were being
assessed. Importantly, the Federal Register [28] attributed their view that paracetamol has “a wide range of safety”
to laboratory animal studies showing that the lethal dose of paracetamol is significantly greater than the dose
administered to humans. Unfortunately, studies had not been conducted at that time showing that paracetamol
induces permanent neurodevelopmental injury in laboratory animals at far lower doses than the lethal dose [15, 16],
similar to doses administered to infants and children.
Discussion:
Our initial search of the PubMed® Database and review of more than 3000 titles and abstracts yielded 218
papers making claims that paracetamol is safe for infants and children when used as directed. Claims of safety in
those 218 papers were traced back to 103 articles shown in Table 2, but less than 20 of those were cited more than
twice, indicating that a limited number of studies are considered key or cornerstone to the view that paracetamol is
safe for use in infants or children.
This study confirms the view that paracetamol use in infants and children is widely thought to be safe when
used as directed, without reservations or caveats. The fact that 27 out of 103 citations did not, in fact, demonstrate
safety or make safety claims might suggest that the safety of paracetamol is taken for granted, and is not carefully
considered. This view is supported by the observation that one popular citation for safety does not exist in the
literature.
Despite apparently being taken for granted, this study demonstrates that the drug was never shown to be safe for
neurodevelopment. This conclusion is consistent with emerging studies showing a connection between paracetamol
use during development and long term neuropsychiatric disfunction as described in the Introduction. This conclusion
is also consistent with emerging studies in animal models showing exquisite sensitivity of long-term behavior to
early life exposure to paracetamol at near-therapeutic doses.
Although not the intended purpose of this systematic review, it demonstrated that paracetamol has been proven
safe for liver function in infants and in small children, even at doses higher than those currently recommended.
During the course of this review, an assumption was repeatedly encountered: because the target of paracetamol
toxicity in adults is the liver, demonstration of safety in infants and children need only be tested in the liver. This
assumption was/is held despite the fact that the target tissue for drug function is in the central nervous system, not
the liver. A similar assumption has proven tragically fatal in the past, when it was assumed that metabolism of the
Page 19 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
antibiotic chloramphenicol was the same in infants as in adults. In that case, administration of the drug in infants led
to a number of deaths [31-33] before the problem was identified.
References
1. Parker, W., et al., The role of oxidative stress, inflammation and acetaminophen exposure from birth to
early childhood in the induction of autism. Journal of International Medical Research, 2017. 45(2): p. 407-
438.
2. Stergiakouli, E., A. Thapar, and G. Davey Smith, Association of Acetaminophen Use During Pregnancy
With Behavioral Problems in Childhood: Evidence Against Confounding. JAMA Pediatr, 2016. 170(10): p.
964-970.
3. Brandlistuen, R.E., et al., Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled
cohort study. International Journal of Epidemiology, 2013. 42(6): p. 1702-1713.
4. Liew, Z., et al., Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders.
JAMA Pediatr, 2014. 168(4): p. 313-20.
5. Liew, Z., et al., Paracetamol use during pregnancy and attention and executive function in offspring at age
5 years. Int J Epidemiol, 2016. 45(6): p. 2009-2017.
6. Thompson, J.M., et al., Associations between acetaminophen use during pregnancy and ADHD symptoms
measured at ages 7 and 11 years. PLoS One, 2014. 9(9): p. e108210.
7. Liew, Z., et al., Prenatal Use of Acetaminophen and Child IQ: A Danish Cohort Study. Epidemiology,
2016. 27(6): p. 912-8.
8. Liew, Z., et al., Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in
childhood: A Danish national birth cohort study. Autism Res, 2016. 9(9): p. 951-8.
9. Avella-Garcia, C.B., et al., Acetaminophen use in pregnancy and neurodevelopment: attention function and
autism spectrum symptoms. Int J Epidemiol, 2016. 45(6): p. 1987-1996.
10. Skovlund, E., et al., Language competence and communication skills in 3-year-old children after prenatal
exposure to analgesic opioids. Pharmacoepidemiol Drug Saf, 2017. 26(6): p. 625-634.
11. Vlenterie, R., et al., Neurodevelopmental problems at 18 months among children exposed to paracetamol in
utero: a propensity score matched cohort study. Int J Epidemiol, 2016. 45(6): p. 1998-2008.
12. Ystrom, E., et al., Prenatal Exposure to Acetaminophen and Risk of ADHD. Pediatrics, 2017. 140(5).
13. Ji, Y., et al., Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of
Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry,
2019: p. 1-11.
14. Bittker, S.S. and K.R. Bell, Acetaminophen, antibiotics, ear infection, breastfeeding, vitamin D drops, and
autism: an epidemiological study. Neuropsychiatr Dis Treat, 2018. 14: p. 1399-1414.
15. Philippot, G., et al., Adult neurobehavioral alterations in male and female mice following developmental
exposure to paracetamol (acetaminophen): characterization of a critical period. J Appl Toxicol, 2017.
37(10): p. 1174-1181.
16. Viberg, H., et al., Paracetamol (Acetaminophen) Administration During Neonatal Brain Development
Affects Cognitive Function and Alters Its Analgesic and Anxiolytic Response in Adult Male Mice.
Toxicological Sciences, 2013. 138(1): p. 139-147.
17. Hay-Schmidt, A., et al., Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs
masculinisation of male brain and behaviour. Reproduction, 2017. 154(2): p. 145-152.
18. Schultz, S.T., et al., Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic
disorder. The results of a parent survey. Autism, 2008. 12(3): p. 293-307.
19. Frisch, M. and J. Simonsen, Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year-old
boys: national cohort study in Denmark. Journal of the Royal Society of Medicine, 2015. 108(7): p. 266-
279.
20. Goin-Kochel, R.P., S.S. Mire, and A.G. Dempsey, Emergence of autism spectrum disorder in children from
simplex families: relations to parental perceptions of etiology. J Autism Dev Disord, 2015. 45(5): p. 1451-
63.
21. Mercer, L., et al., Parental perspectives on the causes of an autism spectrum disorder in their children. J
Genet Couns, 2006. 15(1): p. 41-50.
Page 20 of 25Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 November 2020 doi:10.20944/preprints202011.0173.v1
22. Lesko, S.M. and A.A. Mitchell, The safety of acetaminophen and ibuprofen among children younger than
two years old. Pediatrics, 1999. 104(4): p. e39.
23. Perrott, D.A., et al., Efficacy and safety of acetaminophen vs ibuprofen for treating children's pain or fever:
a meta-analysis. Arch Pediatr Adolesc Med, 2004. 158(6): p. 521-6.
24. Vijarnsorn, C., et al., Postoperative fever and major infections after pediatric cardiac surgery. J Med
Assoc Thai, 2012. 95(6): p. 761-70.
25. Temple, A.R., et al., Comparison of the Efficacy and Safety of 2 Acetaminophen Dosing Regimens in
Febrile Infants and Children: A Report on 3 Legacy Studies. The journal of pediatric pharmacology and
therapeutics : JPPT : the official journal of PPAG, 2017. 22(1): p. 22-32.
26. Shepherd, M. and R. Aickin, Paracetamol versus ibuprofen: a randomized controlled trial of outpatient
analgesia efficacy for paediatric acute limb fractures. Emerg Med Australas, 2009. 21(6): p. 484-90.
27. Temple, A.R., Review of comparative antipyretic activity in children. Am J Med, 1983. 75(5a): p. 38-46.
28. Federal Register. 1977. 42(131): p. 35366-35413.
29. Tarlin, L., et al., A comparison of the antipyretic effect of acetaminophen and aspirin. Another approach to
poison prevention. Am J Dis Child, 1972. 124(6): p. 880-2.
30. Steele, R.W., et al., Oral Antipyretic Therapy: Evaluation of Aspirin-Acetaminophen Combination.
American Journal of Diseases of Children, 1972. 123(3): p. 204-206.
31. Lischner, H., et al., An outbreak of neonatal deaths among term infants associated with administration of
chloramphenicol. The Journal of Pediatrics, 1961. 59(1): p. 21-34.
32. Sutherland, J.M., Fatal cardiovascular collapse of infants receiving large amounts of chloramphenicol.
AMA J Dis Child, 1959. 97(6): p. 761-7.
33. Burns, L.E., J.E. Hodgman, and A.B. Cass, Fatal circulatory collapse in premature infants receiving
chloramphenicol. N Engl J Med, 1959. 261: p. 1318-21.
34. Hämäläinen, M.L., et al., Ibuprofen or acetaminophen for the acute treatment of migraine in children: a
double-blind, randomized, placebo-controlled, crossover study. Neurology, 1997. 48(1): p. 103-7.
35. Rumack, B.H., Aspirin versus acetaminophen: a comparative view. Pediatrics, 1978. 62(5 Pt 2 Suppl): p.
943-6.
36. Litalien, C. and E. Jacqz-Aigrain, Risks and benefits of nonsteroidal anti-inflammatory drugs in children: a
comparison with paracetamol. Paediatr Drugs, 2001. 3(11): p. 817-58.
37. Drwal-Klein, L.A. and S.J. Phelps, Antipyretic therapy in the febrile child. Clin Pharm, 1992. 11(12): p.
1005-21.
38. Sullivan, J.E. and H.C. Farrar, Fever and antipyretic use in children. Pediatrics, 2011. 127(3): p. 580-7.
39. Temple, A.R., Pediatric dosing of acetaminophen. Pediatr Pharmacol (New York), 1983. 3(3-4): p. 321-7.
40. Lesko, S.M. and A.A. Mitchell, An assessment of the safety of pediatric ibuprofen. A practitioner-based
randomized clinical trial. Jama, 1995. 273(12): p. 929-33.
41. Society, C.P., Acetaminophen and ibuprofen in the management of fever and mild to moderate pain in
children Pediatr Child Health, 1998. 3(4): p. 273-274.
42. American Academy of Pediatrics Committee on Drugs: Commentary on acetaminophen. Pediatrics, 1978.
61(1): p. 108-12.
43. Lovejoy, F.H., Jr., Aspirin and acetaminophen: a comparative view of their antipyretic and analgesic
activity. Pediatrics, 1978. 62(5 Pt 2 Suppl): p. 904-9.
44. Prescott, L.F., Paracetamol overdose, in Paracetamol (acetaminophen): A Critical Bibliographic Review.
1996, Taylor & Francis Ltd. p. 451.
45. Lesko, S.M. and A.A. Mitchell, Renal function after short-term ibuprofen use in infants and children.
Pediatrics, 1997. 100(6): p. 954-7.
46. Anderson, B.J., et al., Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population
analysis. Paediatr Anaesth, 2005. 15(4): p. 282-92.
47. Zuppa, A.F., et al., Safety and population pharmacokinetic analysis of intravenous acetaminophen in
neonates, infants, children, and adolescents with pain or Fever. J Pediatr Pharmacol Ther, 2011. 16(4): p.
246-61.
48. Sutton, E. and L.F. Soyka, How safe is acetaminophen? Some practical cautions with this widely used
agent. Clin Pediatr (Phila), 1973. 12(12): p. 692-6.
49. Yaffe, S.J., Comparative Efficacy of Aspirin and Acetaminophen in the Reduction of Fever in Children.
Archives of Internal Medicine, 1981. 141(3): p. 286-292.
50. Depré, M., et al., Tolerance and pharmacokinetics of propacetamol, a paracetamol formulation for
intravenous use. Fundam Clin Pharmacol, 1992. 6(6): p. 259-62.
Page 21 of 25You can also read