Perception of Bradyrhizobium japonicum Nod factor by soybean Glycine max (L.) Merr. root hairs under abiotic stress conditions
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Journal of Experimental Botany, Vol. 55, No. 408, pp. 2641–2646, December 2004
doi:10.1093/jxb/erh265 Advance Access publication 10 September, 2004
RESEARCH PAPER
Perception of Bradyrhizobium japonicum Nod
factor by soybean [Glycine max (L.) Merr.] root
hairs under abiotic stress conditions
H. M. Duzan, X. Zhou, A. Souleimanov and D. L. Smith*
Department of Plant Science, McGill University, Macdonald Campus, 21111 Lakeshore Road,
Ste. Anne de Bellevue, Quebec, Canada H9X 3V9
Received 21 May 2004; Accepted 24 July 2004
Downloaded from http://jxb.oxfordjournals.org/ by guest on July 22, 2015
Abstract Introduction
Suboptimal growth conditions, such as low rhizosphere Successful rhizobia–legume symbioses depend on efficient
temperature, high salinity, and low pH can negatively plant root nodulation and subsequent N2 fixation. During
affect the rhizobia–legume symbioses, resulting in the early stages of infection and nodule organogenesis,
poor nodulation and lower amounts of nitrogen fixed. bacteria attach to the plant roots in response to attractants
Early stages of the Bradyrhizobium japonicum–soybean exuded by the roots, mainly flavonoids, which activate the
[Glycine max (L.) Merr.] symbiosis, such as excretion of bacterial NodD protein, causing transcription of the bacter-
genistein (the plant-to-bacteria signal) and infection ial nod genes. Expression of the nod genes results in
initiation can be inhibited by abiotic stresses; however, synthesis of bacteria-to-plant signal molecules, or Nod
the effect on early events modulated by Nod factors factors. These compounds are composed of tri- to penta-
(bacteria-to-plant signalling), particularly root hair de- chitin backbones and possess an N-acyl group at the non-
formations is unknown. Thus, the objective of this study reducing end and a variety of substitutions along the chitin
was to evaluate the perception of Nod factor by soybean backbone. Differences among Nod factors specify compati-
root hairs under three stress conditions: low tempera- bility between macrosymbiont and microsymbiont species
ture, low pH, and high salinity. Three experiments were (Schultze and Kondorosi, 1998). The application of an
conducted using a 1:1 ratio of Nod Bj-V (C18:1, MeFuc) appropriate Nod factor to soybean [Glycine max (L.) Merr.]
and Nod Bj-V (Ac, C16:0, MeFuc). Nod factor induced four roots triggers responses such as root hair deformation and
types of root hair deformation (HAD), wiggling, bulging, can cause the initiation of nodule primordia or the forma-
curling, and branching. Under optimal experimental tion of complete nodule structures (Carlson et al., 1993;
conditions root hair response to the three levels of Stokkermans et al., 1995). It is now known that the root
Nod factor tested (1026, 1028, and 10210 M) was dose- hair deformation elicited by Nod factors involves signal
dependent. The highest frequency of root hair deforma- transduction through phospholipase C and D (Hartog et al.,
tions was elicited by the 1026 M level. Root hair de- 2001). Recently, the role of phospholipase D in root hair
formation decreased with temperature (25, 17, and morphogenesis was confirmed in Arabidopsis thaliana
15 8C), low pH, and high salinity. Nod factor concentra- (Ohashi et al., 2003).
tion did not interact with either low temperature or pH. Abiotic stress factors, such as salinity, low pH, and low
However, salinity strongly inhibited HAD responses to root-zone temperature can cause poor nodulation in the
increases in Nod factor concentration. Thus, the add- presence of otherwise compatible symbionts. Early events
ition of higher levels of Nod factor is able to overcome the in the symbiosis such as signal production and excretion,
effects of low pH and temperature stress, but not salinity. rhizobial attachment, root hair curling, infection thread
formation, and nodule initiation, are particularly sensitive
Key words: Abiotic stress, Nod factor, root hair deformation, to these stresses (Tu, 1981; McKay and Djordjevic, 1993;
soybean. Zhang and Smith, 1996; Hungria and Stacey, 1997;
* To whom correspondence should be addressed. Fax: +1 514 398 7897. E-mail: Donald.Smith@McGill.Ca
Abbreviations: HAD, root hair deformation; LCO, lipo-chito-oligosaccharide.
Journal of Experimental Botany, Vol. 55, No. 408, ª Society for Experimental Biology 2004; all rights reserved2642 Duzan et al.
Hungria and Vargas, 2000; HM Duzan, A Souleimanov, 30.71
DL Smith, unpublished data).
During the early stages of symbiosis, the first visual 3.30
32.56
response induced by the Nod factor in the presence of
rhizobia is root hair curling. Under optimal growth con- 3.20
X 10-2 volts
ditions, the addition of purified Nod factor to legume root
3.10
systems induces diverse types of root hair deformation,
including, wiggling, curling, bulging, and branching 3.00
(Heidstra et al., 1994; Cullimore et al., 2001; Kelly and
Irving, 2002). However, how this phenomenon is affected 2.90
by abiotic stress factors is not known. Thus, the biological
activity of an equivalent ratio of Nod Bj-V (C18:1, MeFuc) 30.0 30.5 31.0 31.5 32.0 32.5 33.0
and Nod Bj-V (Ac, C16:0, MeFuc) extracted and purified Retention time (min)
from B. japonicum and added to soybean [Glycine max (L.)
Merr.] roots under three abiotic stress conditions: salinity, Fig. 1. HPLC profile of n-butanol extract from Bradyrhizobium
japonicum. Nod Bj-V (C18:1, MeFuc) with a retention time of 30.71
low pH, and low temperature was evaluated. min, Nod Bj-V (Ac, C16:0, MeFuc) with a retention time of 32.56 min.
Downloaded from http://jxb.oxfordjournals.org/ by guest on July 22, 2015
Materials and methods Root hair deformation assay
Soybean (cv. OAC Bayfield) seeds were surface-sterilized in 2%
Bacterial culture sodium hypochlorite for 2 min and washed thoroughly, several times,
Bradyrhizobium japonicum 532C was obtained from Liphatech Inc. with distilled sterilized water. Two seeds were placed on each Petri
(Milwalkee, WI, USA). This strain was selected as it is widely used in plate, containing 10–20 ml 1.5% water agar, and incubated in the
commercial B. japonicum inoculants in Canada and the US. The dark at room temperature. After 5–7 d, lateral roots of similar length
culture was grown at 28 8C in 250 ml yeast mannitol broth (YEM) and showing abundant root hairs were selected for uniformity and
with shaking at 150 rpm for 4–6 d on an incubator orbital shaker aseptically excised and placed on slides containing equivalent
(model 4580, refrigerated console, Forma Scientific Inc. USA), and concentrations of Nod Bj-V (C18:1, MeFuc) and Nod Bj-V (Ac,
thereafter subcultured into 2.0 l of YEM medium. After 7 d of C16:0, MeFuc). The slides were kept in a closed moist chamber for
subculture (OD620 0.4–0.6), bacterial cultures were induced for Nod 24 h. Roots were fixed with a staining solution (Prithiviraj et al.,
factor synthesis and production through the addition of 5 lM 2000). Root hair deformations were observed using light microscopy
genistein and incubated for an additional 48–96 h. (Jenalumar, Jena Instruments Ltd, Germany) with observations
focused on the root hair susceptible zone (Stokkermans et al.,
Nod factor extraction and purification 1995). Each experimental unit had at least four lateral roots, on the
same slide. Each treatment had three replicates. For root hair
The resulting 2.0 l of culture was extracted with 40% HPLC-grade counting, the microscope field consisted of the entire length of the
1-butanol by shaking the mixture for 5–10 min and then allowing the root hair zone of each root. The counts from all fields were added
two phases to separate for 24 h. The organic phase (butanol layer) was together for the determination of the percentage of root hairs
collected and evaporated at 80 8C in a Yamato RE500 Rotary deformed. The same microscopic observations were also made for
evaporator (Yamato Scientific American Inc., NY, USA). The final the control treatment. Roots showing substantial frequencies of
volume, 2–3 ml, was dissolved in 4 ml of 18% of acetonitrile and overlapping root hairs were not used for data collection as this
stored in the dark in glass tubes, at 4 8C for 24 h. Samples were overlapping tended to cause root hair deformation by itself; root hair
centrifuged for 10 min at 12 000 g, and the supernatant was collected deformations caused by Nod factor were considered only in root
for HPLC analysis. hairs not entangled by others (Truchet et al., 1985). With the
For analytical purposes, 200 ll of the Nod factor extract were exceptions just noted, all of the root hairs in each microscopic field
injected into a Waters HPLC system (Waters Associates Inc., were counted.
Milford, MA) consisting of a model 712 WISP, two model 510
pumps, and a model 441 UV detector operating at 214 nm. Soybean root hair response to Nod factor treatment under
Separation was carried out with a Vydac C18 reversed-phase column
salinity, low pH, and low incubation temperature stress
(5 lm, 463250 mm, Vydac, USA). To elute Nod factor from the
column, a programme of acetonitrile and water gradients was used: conditions
18% acetonitrile (10 min), a linear gradient from 18% to 60% Three studies were conducted to evaluate how soybean root hairs
acetonitrile (20 min), and a linear gradient from 60% to 100% respond to exogenous Nod factor applications under three abiotic
acetonitrile (5 min). A chromatographic peak with a retention time of stress factors: salinity, low pH, and low incubation temperatures. In
30.71 min was identified as Nod Bj-V (C18:1, MeFuc) by comparing each experiment, the treatments consisted of factorial combinations
its retention time with a standard of this Nod factor (kindly provided of a control where only distilled sterilized H2O was used, and three
by Professor G Stacey, Center for Legumes Research, University of Nod factor concentrations (10ÿ6, 10ÿ8, 10ÿ10 M) where the Nod
Tennessee, Knoxville, USA). A second peak with a retention time factor consisted of a 1:1 mixture of Bj-V (C18:1, MeFuc) and Nod
of 32.56 min was also purified by preparative HPLC. Mass Bj-V (Ac, C16:0, MeFuc). The LCO (lipo-chito-oligosaccharide)
spectrometry analysis (electrospray in negative mode) carried out material was freeze-dried and dissolved in distilled sterilized water to
at the Biomedical Mass Spectrometry Unit, McGill University produce the required LCO solutions. Modifications were applied,
(Dr O Mamer) allowed this peak to be identified as Nod Bj-V (Ac, based on the type of stress to be tested, as described in the following
C16:0, MeFuc) (Fig. 1). paragraphs.Nod factor perception by root hairs 2643
To test the effect of low incubation temperature on Nod factor Biological activity of Nod factor at low incubation
bioactivity, a study was carried out with three incubation tempera- temperature
tures (15, 17, and 25 8C). The range of incubation temperatures was
chosen based on the finding of Zhang and Smith (1994), where The frequency of root hair deformation was dose-dependent.
a progressive delay of 1–2 d 8Cÿ1 in the early stages of the soybean In general, 10ÿ6 M Nod factor provoked the highest response
infection process was reported as temperature declined from 25 8C (28.5%) of root hair deformations over a range of tested Nod
(optimum) to 17.5 8C root-zone temperature (RZT). Between 17.5 8C factor. As the Nod factor concentration declined, marked
and 15 8C the delay was much larger, at 7–10 d 8Cÿ1. Solutions of
prechilled Nod factor (10ÿ6, 10ÿ8, 10ÿ10, and 0.0 M) were prepared reductions in HAD were observed, where 20.6% and 14.5%
as previously described. In the control treatment, distilled sterilized were the lowest average values of root hair deformation
water was applied to the lateral roots. observed for Nod factor treatments at 10ÿ8 and 10ÿ10 M,
To test the effect of low pH on Nod factor bioactivity, Nod respectively (Fig. 3). There was no interaction between Nod
factor solutions (10ÿ6, 10ÿ8, 10ÿ10 M) were adjusted to two levels of factor and incubation temperature. Suboptimal incubation
pH (4 and 5) using HCl. Two control treatments were used in this
study, Nod factor solution with a control pH of 6 and, as in the first temperature markedly affected the response of soybean root
study, a distilled sterilized water control. hairs to Nod factor. As the temperature decreased, lower
To test the effect of salinity on Nod factor bioactivity, two frequencies of root hair deformation were detected at 17 8C,
concentrations of NaCl (100 and 200 mM) were prepared by HAD was 82% of the control (plant roots incubated at 25 8C),
dissolving the NaCl in distilled sterilized water and mixing this with while at 15 8C this was only 64% (Fig. 3).
Nod factor solutions; the final volume was adjusted according to the
assigned concentrations. Two controls were applied to the lateral
roots: distilled sterilized water, NaCl-free Nod factor solutions. Biological activity of Nod factor under low pH
Downloaded from http://jxb.oxfordjournals.org/ by guest on July 22, 2015
In the three studies, soybean lateral roots of similar lengths and conditions
showing abundant fluffy root hair growth were aseptically excised
Similar to the findings with low temperature stress, in these
and placed on microscope slides containing Nod factor solutions at
a pH of 6 for salinity and low temperatures studies. To ensure experiments there was no interaction between Nod factor
complete coverage of root hairs, extra Nod factor solution was added and pH. Over all, 51% of soybean root hairs were deformed
to each specimen which was then incubated in the dark in a moist when treated with 10ÿ6 M Nod factor at pH 6. Root hair
chamber at 25 8C for salinity and low pH stress factors, while for low deformation decreased as Nod factor concentration de-
temperature stress experiments, slides were incubated at three
creased; averages of 34% and 19.7% of root hairs were
different temperatures (15, 17, and 25 8C). After 24 h, root hairs
were stained with methylene blue (0.01%) and then scored for HAD deformed by 10ÿ8 and 10ÿ10 M Nod factor, respectively
under the microscope (Prithiviraj et al., 2000). (Fig. 4). The smallest response to Nod factor solution
occurred at pH 4, with 29.8% of root hairs deformed. The
Experimental design and statistical analysis degree of response at pH 5 was 37.8% HAD (Fig. 4).
The experiments were each conducted twice. Because similar data
were observed in both experiments, data were combined and Biological activity of Nod factor under salinity stress
subjected to statistical analysis. In each experiment, the treatments
were the result of factorial combinations of two factors, and the There was an interaction between salinity and Nod factor
treatments were organized following a randomized split-plot design treatment. All Nod factor treatments elicited root hair
with three replicates per treatment. The main plot units consisted of deformation for the control treatment; where the propor-
the temperature, salinity, or pH levels, while Nod factor concen-
tional HAD was 42, 27, and 14% at 10ÿ6, 10ÿ8, 10ÿ10 M,
trations formed the subplots. The percentage deformation data for
Nod factor-treated roots were square root transformed prior to respectively (Fig. 5). The biological activities of NaCl-
statistical analysis (Steel and Torrie, 1980). The control data were amended Nod factor solutions were markedly diminished at
excluded from the statistical analysis because root hair deformation the two salt concentrations tested (200 and 100 mM) and
was not detectable in this treatment. The Statistical Analysis System there was no difference between them.
(SAS Institute Inc., 1990) was used for analysis of variance. When
a significant treatment effect (P2644 Duzan et al.
Downloaded from http://jxb.oxfordjournals.org/ by guest on July 22, 2015
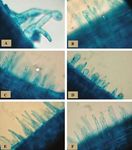
Fig. 2. Types of root hair deformations manifested by Nod factor treatments. (A) Root hair branching (magnification 3615). (B) Root hair curling
(magnification 3308). (C, D) Root hair bulging (magnification 3288). (E) Untreated root hairs (control, magnification 3462). (F) Root hair wiggling
(magnification 3384).
An attempt was made to mimic stress effects, in vivo, on low temperature on the bioactivity of Nod factor. Pre-
early stages of the perception of Nod factor in the soybean– viously, the negative effect of low growth temperature on
rhizobia association (Lhuissier et al., 2001). Because of the Nod factor production by B. japonicum 532C and US-
size of soybean seeds and the nature and number of DA110 was addressed, a prior step in the symbiotic
treatments required, the method described by Prithiviraj recognition process (HM Duzan, A Souleimanov, DL
et al. (2000) was followed, which has been proven to Smith, unpublished data). These data corroborate the low
trigger a range of types and appreciable frequencies of temperature inhibitory effect on the early stages of nodu-
soybean root hair deformation when compared with other lation, specifically, on root hair curling, the first visible step
methods (Heidstra et al., 1994; Stokkermans et al., 1995) in nodulation, and the step immediately following Nod
and the data from the controls showed that good frequen- signal excretion by rhizobia in the presence of a compatible
cies of HAD were observed with this method. These data legume partner (Zhang and Smith, 1994).
are in agreement with previous studies with respect to the There was a reduction in Nod factor activity under low
frequencies of root hair deformation evoked by a variety of pH conditions. This is in agreement with previous obser-
Nod factors and leguminous plants. vations regarding the low pH-sensitivity of the early steps
The impact of suboptimal root-zone temperatures on in the nodulation process including root hair deformation,
soybean–bradyrhizobia associations, particularly at early nod gene expression, Nod factor production, and root hair
stages of symbiosis, has been addressed in a number of curling, all of which are probably related to effects on both
studies (Zhang and Smith, 1994, 1995, 1996; Zhang et al., rhizobia and the plant root system (Evans et al., 1980;
1996); evidence is presented here of the negative effects of Richardson et al., 1988; McKay and Djordjevic, 1993;Nod factor perception by root hairs 2645
60
30 c c
( ) Root hair deformations
50
( ) Root hair deformations
b 40
20 b
a 30
a
10 20
10
0
10-10 10-8 10-6 0
Nod factor concentrations (M) 10-10 10-8 10-6
Nod factor concentrations (M)
30
c
50
) Root hair deformations
25 b
) Root hair deformations
b
Downloaded from http://jxb.oxfordjournals.org/ by guest on July 22, 2015
20 40 ab
a
a
15 30
10
20
5
(
10
(
0
15 17 25
0
Temperature (°C) 4 5 6
Fig. 3. (A) Root hair deformations as a function of Nod factor
pH
concentrations. (B) Root hair deformations at three incubation tempera- Fig. 4. (A) Root hair deformations as a function of Nod factor
tures. Bars associated with a different letter are significantly different, by concentrations. (B) Root hair deformations tested at different pH levels.
an ANOVA (P=0.05) protected LSD0.05 test. Bars associated with the same letter are not significantly different, by an
ANOVA (P=0.05) protected LSD0.05 test.
Staley, 2003). In white clover, morphological parameters of
root growth and development, such as total length, di-
ameter, tip number, and surface area were not affected by 60
low soil pH, while rhizobia proliferation and function were, Nod factors 10-6 M
suggesting that the inhibitory effect of low pH occurs at Nod factors 10-8 M
) Root hair deformations
50 Nod factors 10-10 M
the microbial level, affecting nodule primordia initiation
a
(Staley, 2002, 2003). There may be other, less direct, 40
environmental effects on nodulation due to low pH, such as
the limitation of the amount of Nod factor excreted by the 30 b
microsymbiont through the reduced availability of phos-
phate (McKay and Djordjevic, 1993). 20 c
c c c
Salt stress decreased the soybean root hair responses to c c c
Nod factor. This is reasonable as salinity can directly 10
restrict legume root growth, affecting the responses of roots
(
to rhizobia and Nod factor (Tu, 1981). Salinity levels of 0
255 mM and 340 mM NaCl caused shrinkage of soybean 0.0 100 200
root hairs, affecting the early stages of symbiosis, particu- NaCl concentrations (mM)
larly root hair deformation (curling), resulting in an
Fig. 5. The effect of various salt concentrations on Nod factor bio-
inhibition of the infection process (Tu, 1981; Elsheikh, activity. Bars associated with the same letter are not significantly
1998). Minor effects of osmolarity have been reported on different, by an ANOVA (P=0.05) protected LSD0.05 test.2646 Duzan et al.
nodD and nodABC expression in B. japonicum (Wang and in the field. Applied and Environmental Microbiology 59,
Stacey, 1990). 3385–3392.
Minami E, Kouchi H, Carlson R, Cohn J, Kolli V, Day R, Ogawa
In conclusion, as far as the authors are aware, this is the T, Stacey G. 1996. Cooperative action of lipo-chitin nodulation
first study addressing the external response of soybean root signals on the induction of the early nodulin, ENOD2, in soybean
hairs to Nod factor application under abiotic stress con- roots. Molecular Plant–Microbe Interactions 9, 574–583.
ditions. Under controlled environment conditions, and in Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Ruberti I,
the presence of low pH and low temperature stresses, the Morelli G, Aoyama T. 2003. Modulation of phospholipid signal-
ling by GLABRA2 in root-hair pattern formation. Science 300,
degree of root hair deformation increased with increasing 1427–1430.
Nod factor concentration, and diminished as the applied Prithiviraj B, Souleimanov A, Zhou X, Smith DL. 2000. Differ-
stress increased. For low temperature and pH stresses there ential response of soybean (Glycine max (L.) Merr.) genotypes to
was no interaction between Nod factor level and stress lipo-chito-oligosaccharide Nod Bj-V (C18:1, MeFuc). Journal of
level. However, for salinity the increase in root hair Experimental Botany 51, 2045–2051.
Richardson AE, Djordjevic MA, Rolfe BG, Simpson RJ. 1988.
deformation with increasing Nod factor concentration Effects of pH, Ca, and Al on the exudation from clover seedlings of
occurred in the control, but not when salt stress was compounds that induce the expression of nodulation genes in
present. Thus, adding additional Nod factor helped over- Rhizobium trifolii. Plant and Soil 109, 37–47.
come the measured stress effect associated with low SAS Institute Inc. 1990. SAS users guide. Cary, North Carolina:
temperature and low pH, but this was not the case for Statistical Analysis Institute Inc.
Schultze M, Kondorosi A. 1998. Regulation of symbiotic root
salinity. Further studies would be instructive to address the nodule development. Annual Review of Genetics 32, 33–57.
effect of these stresses on Nod factor structure.
Downloaded from http://jxb.oxfordjournals.org/ by guest on July 22, 2015
Staley TE. 2002. Low-level liming affects early white clover
nodulations but not root development in a small-volume acidic
soil model system. Soil Science 167, 211–221.
References Staley TE. 2003. Initial white clover nodulation under saturation
levels of rhizobia relative to low-level liming of an acidic soil. Soil
Carlson RW, Sanjuan J, Bhat R, Glushka J, Spaink HP, Wijfjes Science 168, 540–551.
AHW, van Brussel ANA, Stokkermans TJW, Peters NK, Steel RGD, Torrie JH. 1980. Principles and procedures of
Stacey G. 1993. The structures and biological activities of the statistics: a biometrical approach, 2nd edn. New York, NY:
lipo-oligosacchride nodulation signals produced by type I and II McGraw-Hill Book Co.
strains of Bradyrhizoboium japonicum. Journal of Biological Stokkermans TJW, Ikeshita S, Cohn J, Carlson RW, Stacey G,
Chemistry 268, 18372–18381. Ogawa T, Peters NK. 1995. Structural requirements of synthetic
Cullimore JV, Ranjeva R, Bono JJ. 2001. Perception of lipo- and natural product lipo-chitin oligosaccharides for induction
chito-oligosaccharide Nod factors in legumes. Trends in Plant of nodule primordia on Glycine soja. Plant Physiology 108,
Science 6, 24–30. 1587–1595.
Elsheikh EAE. 1998. Effects of salt on rhizobia and bradyrhizobia: Truchet G, Debelle F, Vasse J, Terzaghi B, Garnerone AM,
a review. Annals of Applied Biology 132, 507–524. Rosenberg C, Batut J, Maillet F, Denarie J. 1985. Identification
Evans LS, Lewin KF, Vella FA. 1980. Effect of nutrient medium pH of a Rhizobium meliloti pSym2011 region controlling the host
on symbiotic nitrogen fixation by Rhizobium leguminosarum and specificity of root hair curling and nodulation. Journal of Bacte-
Pisum sativum. Plant and Soil 56, 71–80. riology 164, 1200–1210.
Hartog MD, Musgrave A, Munnik T. 2001. Nod factor-induced Truchet G, Roche P, Lerouge P, Vasse J, Camut S, de Billy F,
phosphatidic acid and diacylglycerol pyrophosphate formation: Prome J-C, Denarie J. 1991. Sulphated lipo-oligosaccharide
a role for phospholipase C and D in root hair deformation. The signals of Rhizobium meliloti elicit root nodule. Nature 351,
Plant Journal 25, 55–65. 670–673.
Heidstra R, Geurts R, Franssen H, Spaink HP, van Kammen A, Tu JC. 1981. Effect of salinity on rhizobium–root-hair interaction,
Bisseling T. 1994. Root hair deformation activity of nodulation nodulation and growth of soybean. Canadian Journal of Plant
factors and their fate on Vicia sativa. Plant Physiology 105, Science 61, 231–239.
787–797. Wang SP, Stacey G. 1990. Ammonia regulation of nod genes in
Hungria M, Stacey G. 1997. Molecular signals exchanged between Bradyrhizobium japonicum. Molecular and General Genetics
host plants and rhizobia: basic aspects and potential application in 223, 329–331.
agriculture. Soil Biology and Biochemistry 29, 819–830. Zhang F, Smith DL. 1994. Effects of low root zone temperature on
Hungria M, Vargas MAT. 2000. Environmental factors affecting N2 the early stages of symbiosis establishment between soybean
fixation in grain legumes in the tropics, with an emphasis on Brazil. (Glycine max. (L.) Merr) and Bradyrhizobium japonicum. Journal
Field Crops Research 65, 151–164. of Experimental Botany 45, 1467–1473.
Kelly MN, Irving HR. 2002. Nod factors activate both heterotri- Zhang F, Smith DL. 1995. Preincubation of Bradyrhizobium
meric and monomeric G-proteins in Vigna unguiculata (L.) Walp. japonicum with genistein accelerates nodule development of
Planta 216, 674–685. soybean at suboptimal root zone temperatures. Plant Physiology
Lhuissier FGP, De Ruijter NCA, Sieberer BJ, Esseling JJ, 108, 961–968.
Emons AMC. 2001. Time-course of cell biological events evoked Zhang F, Smith DL. 1996. Genistein accumulation in soybean
in legume root hairs by Rhizobium Nod factors: state of the art. (Glycine max (L.) Merr.) root systems under suboptimal root zone
Annals of Botany 87, 289–302. temperatures. Journal of Experimental Botany 47, 785–792.
McKay IA, Djordjevic MA. 1993. Production and excretion of Zhang F, Charles TC, Pan B, Smith DL. 1996. Inhibition of the
nod metabolites by Rhizobium leguminosarum bv. trifolii are expression of Bradyrhizobium japonicum nod genes at low
disrupted by the same environmental factors that reduce nodulation temperatures. Soil Biology and Biochemistry 28, 1579–1583.You can also read