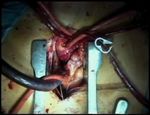
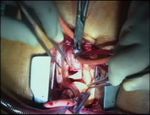
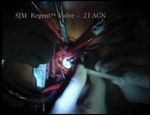
Supra-annular aortic valve replacement with a mechanical prosthesis
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
doi:10.1510/mmcts.2004.000646
Supra-annular aortic valve replacement with a
mechanical prosthesis
Marko Turina*
University Hospital, Rämistrasse 100, 8091 Zurich, Switzerland
Supra-annular placement of the aortic valve prosthesis is primarily used in small aortic annu-
lus to prevent a patient-prosthesis mismatch. The most important surgical steps are: careful
removal of the diseased valve including annular decalcification; assessment of the prosthesis
seating to guarantee free movement of leaflets and to prevent obstruction of coronary ori-
fices; implantation with interrupted stitches placed infra-annularly, with the prosthesis being
seated in a supra-annular position. If necessary, a sub-annular resection of asymmetric
septal hypertrophy should be carried out prior to placement of the prosthesis.
Keywords: Aortic prosthesis; Mechanical valve; Patient-prosthesis mismatch; Supra-annular
placement
Introduction pentier in 1981.1 By placing the valve prosthesis
above the annulus, the narrowing of the left ventricular
Aortic valve replacement is performed to replace a outflow by the sewing ring of the prosthesis is mini-
diseased valve with a new, competent one with min- mized, resulting in improved hemodynamic perform-
imal pressure gradient. Many prosthetic valves, ance. Some newer mechanical prosthetic valves are
mechanical and biological, are not free of some designed for supra-annular placement, to be used
obstruction to the blood flow, and a residual gradient in a narrow aortic orifice with a diameter below 21–
is common, especially in small aortic orifices. In 1978 23 mm (MMCTSLink 78). This procedure depicts the
Rahimtoola coined the term ‘patient-prosthesis mis- replacement of the stenotic aortic valve with Regent
match’ w1x to describe the condition where an impedi- SJM prosthesis (MMCTSLink 79).
ment to the blood outflow in patients with small aortic
prosthesis results in incomplete regression of the left
ventricular hypertrophy, with residual symptoms and Surgical technique
possibly a decreased life expectancy. As a lower level
The patient is a 55-year-old female with severe aortic
of acceptable valve opening area a value of 0.85
stenosis and only minimal aortic incompetence (Video
cm2/m2 is generally acknowledged w2x, although the
1). The operation is performed via a short median ster-
concept of patient-prosthesis mismatch has been
notomy (Video 2), which gives adequate exposure to
questioned recently, possibly due to the improved
the ascending aorta and aortic valve, and allows stan-
performance of newer mechanical and biological
dard cannulation without specially designed equip-
valves w3–5x.
ment and cannulas. After cannulation of the as-
The original idea of supra-annular placement of the cending aorta, with a long cannula whose tip is placed
valve prosthesis was described and patented by Car- into the descending aorta to minimize embolization
(MMCTSLink 80), and of the right atrium by a two-
* Corresponding author: Tel.: q41-44-255 3298; fax q41-44-255
4446. 1
Carpentier A, Lane E. Original patent for supra-annular valve
E-mail: marko.turina@usz.ch replacement. http://www.freepatentsonline.com/4451936.html
䉷 2005 European Association for Cardio-thoracic Surgery 1M. Turina / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2004.000646
Video 1. Trans-esophageal echocardiography demonstrating sub-
Video 4. Calcified aortic valve is removed by sharp dissection using
stantial calcifications in a degenerative aortic valve disease with
a knife, heavy scissors and rongeur, followed by careful decalcifi-
predominant stenosis. The aortic annulus is small, measured at
cation of the annulus. A sponge is placed into the left ventricular
21 mm – 䉷 St. Jude Medical Inc. Courtesy of St. Jude Medical. All
cavity to catch any calcific debris, and a thorough irrigation of the
rights reserved.
area is performed, again to catch any calcified material becoming
loose during valve removal – 䉷 St. Jude Medical Inc. Courtesy of
St. Jude Medical. All rights reserved.
Video 2. Surgery is performed through a small upper mid-sternal
incision reaching to the fourth intercostal space. Good exposure of
the aortic valve is achieved, and all necessary cannulations (aorta,
right atrium, coronary sinus and left ventricular vent) are possible – Video 5. Sizing of the annulus, using first a plain numbered sizer,
䉷 St. Jude Medical Inc. Courtesy of St. Jude Medical. All rights and later a ring sizer which delineates the position of the prosthesis
reserved. after implantation. Coronary arteries should be well visible at this
stage – 䉷 St. Jude Medical Inc. Courtesy of St. Jude Medical. All
rights reserved.
cross-clamped and antegrade cold cardioplegia start-
ed (Video 3).
After achieving a good electro-mechanical standstill,
retrograde cardioplegia is started and the aorta is
opened with a conventional hockey-stick incision
reaching into the non-coronary sinus. Stenotic, de-
generated aortic valve is removed (Video 4), and care-
Video 3. Standard cannulation for cardio-pulmonary bypass is per-
ful decalcification of the annulus is performed. A
formed, with a long arterial cannula introduced through the ascend-
ing aorta, reaching beyond the subclavian artery into the surgical sponge is placed into the left ventricle during
descending aorta, and a conventional two-stage cannula is placed this procedure to capture calcific debris which is un-
into the right atrium. In cardiopulmonary bypass, left ventricular vent avoidable at this stage. Special care is taken when
is introduced through the upper right pulmonary vein, and antegra- working in the left and non-coronary sinuses, to pre-
de and retrograde cardioplegia cannulas are placed. With a com-
vent detachment of aorta from the fibrous skeleton of
petent aortic valve, cardioplegia is started in antegrade fashion
through the ascending aorta; after achieving a good standstill, ret- the heart. Calcifications often extend into the outflow
rograde cardioplegia is continued, followed by continuous retro- tract and onto the anterior mitral leaflet; judicious
grade administration of cold (16 8C) oxygenated blood – 䉷 St. Jude removal is indicated, taking care to increase the motil-
Medical Inc. Courtesy of St. Jude Medical. All rights reserved. ity and preserve the integrity of the mitral valve; and
to stay away from the membranous septum of the
stage venous cannula (MMCTSLink 81), cardiopul- heart, to prevent injury to the penetrating His-bundle.
monary bypass is instituted and the patient cooled to The subannular region is checked for possible asym-
30 8C. The left ventricle is vented (MMCTSLink 82), metric septal hypertrophy, which should be resected
antegrade and retrograde cardioplegia cannulas are at this stage, because it can cause postoperative gra-
placed (MMCTSLink 83 and MMCTSLink 84), aorta is dient after valve replacement w6,7x.
2M. Turina / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2004.000646
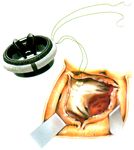
Schematic 2. Infra-annularly placed, pledgetted non-everting mat-
tress sutures, by courtesy of Carbomedics.
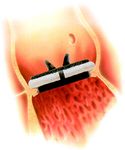
Schematic 1. Difference between intra- and supra-annular place-
ment of the aortic valve prosthesis, by courtesy of Carbomedics 2.
Sizing of the annulus is performed next (Video 5), first
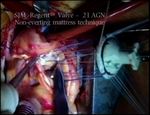
determining the diameter, and later checking the seat- Schematic 3. Schematic implantation technique with interrupted
ing of the prosthesis, especially in relation to the ori- non-everting mattress, by Courtesy of Sorin 3.
fices of coronary arteries and to the aortotomy itself.
Misleading dimensions of the sizers must be kept in sis (Schematic 2), and might reduce the incidence of
mind w8,9x, to select a prosthesis which will actually paraprosthetic leaks w10x. All stitches are placed first,
fit into the annulus. and the prosthesis is kept at a distance of 8–0 cm by
the assistant; the use of two-colored sutures
The prosthesis is implanted using sub-annular, non- (MMCTSLink 7) greatly facilitates the procedure
everting pledgetted mattress stitches (Schematic 1), (Schematic 3). Videos 6 and 7 show the stitches being
which assure a supra-annular seating of the prosthe- placed in the right (Video 6) and left sinuses (Video 7).
2
http://www.carbomedics.com/pdfs/thwhtppr.pdf 3
http://www.sorin-cid.com/bicar_slim_tec.htm.
3M. Turina / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2004.000646
Video 6. Three heavy sutures are passed through the commissures
and traction is applied: this brings the annulus higher into the aor-
totomy and stabilizes the tissue when stitching. Interrupted, double
armed, pledgetted sutures are placed infra-annularly, exiting slightly
above the annulus. Suturing is started in the right coronary sinus;
strong perpendicular traction is applied to each stitch, preparing the
tissue for the placement of the next suture – 䉷 St. Jude Medical
Inc. Courtesy of St. Jude Medical. All rights reserved.
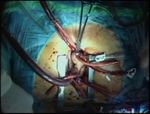
Schematic 4. Supra-annular seating of the prosthesis, by Courtesy
of Sorin.
Video 7. Left coronary commissure sutures are placed next; con-
tinuous retrograde blood administration can be interrupted at this
stage for several minutes, to assure adequate visualization. Again,
strong perpendicular traction is applied to sutures already placed –
䉷 St. Jude Medical Inc. Courtesy of St. Jude Medical. All rights
reserved.
Video 9. Sutures are tied sequentially; two-colored sutures greatly
simplify the task and expedite the procedure. The valve holder is
kept in place and downward pressure is exerted until non-coronary
and left coronary stitches are tied. At this stage, the holder is
removed and residual stitches in the right commissure are tied – 䉷
St. Jude Medical Inc. Courtesy of St. Jude Medical. All rights
reserved.
Video 8. Non-coronary commissure is the last where the sutures
are placed; after completion of the sewing the prosthesis is lowered Last sutures are placed in the non-coronary sinus; the
into position, but the valve holder is kept in place. The assistant
exerts strong downward pressure on the holder to seat the pros-
prosthesis is left in the holder and it is lowered into
thesis, to prevent any undue tension being placed on the sutures the supra-annular position (Schematic 4, and Video 8).
when tying – 䉷 St. Jude Medical Inc. Courtesy of St. Jude Medical.
All rights reserved.
All sutures are tied, with the assistant pressing the
valve holder into the annulus, so then no additional
It is essential to keep the sutures which have been tension has to be placed on the sutures during tying
already placed under constant tension, to elevate the (Video 9). This holder can be usually kept in place until
aortic annulus and stabilize the area for the next sutures in the non-coronary and left coronary sinuses
stitch. Continuous retrograde cardioplegia can be are tied; afterwards the holder has to be removed, and
interrupted for several minutes if blood obscures the a gentle downward pressure on the sewing ring can
field. be exerted by a small hemostat. After all sutures have
4M. Turina / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2004.000646
Video 10. Sutures are cut individually, one by one; they are cut Video 11. After the heart resumes its activity and begins ejecting,
short, practically in the knot, to guarantee free movement of the trans-esophageal echo is repeated, to check leaflet motion and rule
prosthetic leaflets. Leaflet movement is assessed by a special soft- out paravalvular leaks. The probe is left in place to check for ade-
tipped instrument, sub-annular region is inspected for any possible quate de-airing before decannulation – 䉷 St. Jude Medical Inc.
residual material or hypertrophic muscle which might lead to Courtesy of St. Jude Medical. All rights reserved.
reduced leaflet mobility, and coronary orifices are checked with a
right-angle clamp. Warm retrograde cardioplegia is started during
closure of the aortotomy – 䉷 St. Jude Medical Inc. Courtesy of St. Movement of both leaflets is checked by a soft-tipped
Jude Medical. All rights reserved. instrument provided by the manufacturers; with both
leaflets held open the subvalvar region is inspected
been securely tied, they are cut very short, practically for possible tissue residues which might cause pan-
in the knot, to prevent any possible impediment of the nus ingrowth later. Warm retrograde cardioplegia
leaflet motion by too long knot rests (Video 10). (‘hot-shot’) is started, and aortotomy is closed by run-
Table 1. Odds ratio for hospital mortality – reprinted from Astor BC, Kaczmarek RG, Hefflin B, Daley WR. Mortality after aortic valve
replacement: results from a nationally representative database. Ann Thorac Surg 2000;70:1939–1945 (Ref. w12x), with permission from the
Society of Thoracic Surgeons
Characteristics Adjusted odds ratio for 95% Confidence P-value
in-hospital mortality interval
Age (per 10 year) 1.29 1.16–1.43 -0.001
Female (versus male) sex 1.33 1.12–1.59 0.002
Bioprosthetic (versus mechanical) valvea 1.24 1.01–1.53 0.04
Multiple valve positions replaced 1.75 1.32–2.31 -0.001
Replacement of previously implanted 2.26 1.63–3.14 -0.001
prosthetic heart valveb
Concurrent CABG 1.64 1.34–2.01 -0.001
Hospital admission statusc (versus elective
status)
Emergent 1.30 1.02–1.64 0.03
Urgent 1.92 1.53–2.41 -0.001
Comorbid conditions
Rheumatic valve disease 1.10 0.87–1.40 0.043
Heart failure 1.62 1.28–2.05 -0.001
Chronic renal failure 2.57 1.61–4.10 -0.001
Cerebrovascular disease 2.26 1.70–3.00 -0.001
Hospital characteristics
Urban (versus rural) location 0.74 0.28–1.60 0.53
Teaching (versus nonteaching) 1.26 0.99–1.40 0.06
Hospital control (versus government
control)
Private non-profit 0.76 0.54–1.08 0.12
Private for-profit 0.62 0.33–1.17 0.14
Hospital volume (versus low volume)
Medium-low (61–100 procedures) 0.90 0.66–1.24 0.53
Medium-high (101–180 procedures) 0.89 0.66–1.20 0.46
High ()180 procedures) 0.58 0.42–0.81 0.001
ns46,397 (95% Confidence interval 40,005 to 52,789).
a
An estimated 42 patients (7 records) reportedly received both a mechanical and bioprosthetic aortic valve.
b
An estimated 115 patients (36 records) had diagnoses of more than one heart valve failure mode.
c
An estimated 876 patients (164 records) did not specify hospital admission status and were assigned a dummy variable representing
missing admission status. CABGscoronary artery bypass graft.
5M. Turina / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2004.000646
Table 2. Odds ratio for hospital mortality, with major risk factors identifieda – reprinted from Jin R, Grunkemeier GL, Starr A. Validation and
refinement of mortality risk models for heart valve surgery. Ann Thorac Surg 2005;80: 471–479 w13x, with permission from the Society of
Thoracic Surgeons
Coefficients S.E. of coefficients Odds ratio (95% CI) P-value
Age (years) 0.05743 0.009 1.06(1.04,1.08) -0.001
Female 0.3418 0.157 1.41(1.03,1.92) 0.030
BMI -20 or )32 (kg/cm2) 0.6003 0.168 1.82(1.31,2.53) -0.001
Priority of operation
Urgent 0.4547 0.172 1.58(1.126,2.21) 0.008
Emergent 1.723 0.333 5.60(2.918,10.76) -0.001
Salvage 2.452 0.575 11.61(3.76,35.81) -0.001
History of cardiac surgery 0.8210 0.170 2.27(1.628,3.17) -0.001
Peripheral vascular disease 0.5052 0.181 1.66(1.16,2.36) 0.005
COPD 0.5162 0.168 1.68(1.206,2.33) 0.002
Pulmonary hypertension 0.5270 0.162 1.69(1.23,2.33) 0.001
Atrial fibrillation 0.5062 0.171 1.66(1.188,2.32) 0.003
Logarithm of Cr (mg/dl) 1.212 0.196 3.36(2.287,4.93) -0.001
NYHA class 4 0.3555 0.192 1.43(0.98,2.08) 0.064
Concomitant CABG 0.3680 0.162 1.45(1.05,1.98) 0.023
Constant y8.890 0.675
BMIsbody mass index; CIsconfidence interval; COPDschronic obstructive pulmonary disease; Crscreatinine; NYHAsNew York Heart
Association functional class; S.E.sstandard error.
a
Independent risk factors for operative mortality for aortic valve replacement or mitral valve replacement or repair, with or without coronary
artery bypass graft surgery (CABG).
ning monofilament suture (MMCTSLink 50). With the w17,18x, and various interventions to enlarge the annu-
heart de-aired and ejecting, the function of the pros- lus are hardly needed.
thesis is checked by echocardiography (Video 11).
This last step is highly recommended, because it can
References
detect potential technical problems which cannot be
recognized by standard hemodynamic monitoring w1x Rahimtoola SH. The problem of valve prosthesis-
w10,11x. patient mismatch. Circulation 1978;58:20–24.
w2x Pibarot P, Dumesmil JG, Lemieux M, Cartier P,
Métras J, Durand LG. Impact of prosthesis-
patient mismatch on hemodynamic and symp-
Results and discussion tomatic status, morbidity and mortality after aortic
valve replacement with a bioprosthetic heart
valve. J Heart Valve Dis 1998;7:211–218.
Early mortality of aortic valve replacement lies in large w3x Banbury MK, Cosgrove III, DM, White JA,
databanks 4,5,6 between 3 and 4%. It is obviously high- Blackstone EH, Frater RWM, Okies JE. Age and
er in emergency procedures, endocarditis, reopera- valve size effect on the long-term durability of the
tions, and in aged patients, to quote only a few factors Carpentier-Edwards aortic pericardial biopros-
(Tables 1 and 2). Reliable risk calculators are available thesis. Ann Thorac Surg 2001;72:753–757.
for assessing the hazard of aortic valve replacement w4x Medalion B, Blackstone EH, Lytle BW, White J,
in a particular patient w12,13x. Careful attention to Arnold JH, Cosgrove DM. Aortic valve replace-
technical details is still essential, especially in elderly ment: is valve size important? J Thorac Cardio-
patients and in those with small aortic annulus, to pre- vasc Surg 2000;119:963–974.
vent problems which might arise from coronary orifice w5x Aupart M, Simonnot I, Sirinelli A, Meurisse Y,
obstruction w14x or impeded leaflet motion w15,16x. Babuty D, Marchannd M. Pericardial valves in
Supra-annular placement of the prosthesis results in small aortic annuli: ten years’ results. Eur J
very satisfactory hemodynamics, even in small aorta Cardiothorac Surg 1996;10:879–883.
w6x Turina M. Asymmetric septal hypertrophy should
4
http://www.sts.org/documents/pdf/Spring2005STS- be resected during aortic valve replacement. Z
ExecutiveSummary.pdf
5
http://www.ctsnet.org/file/SCTS2000pages66-71General.pdf
Kardiol 1986;75(Suppl 2):198–200.
6
http://www.health.state.ny.us/nysdoh/heart/pdf/2000- w7x Hess OM, Schneider J, Turina M, Carroll JD,
2002_cabg.pdf Rothlin M, Krayenbuehl HP. Asymmetric septal
6M. Turina / Multimedia Manual of Cardiothoracic Surgery / doi:10.1510/mmcts.2004.000646
hypertrophy in patients with aortic stenosis: an refinement of mortality risk models for heart valve
adaptive mechanism or a coexistence of hy- surgery. Ann Thorac Surg 2005;80:471–479.
pertrophic cardiomyopathy? J Am Coll Cardiol w14x Santini F, Pentiricci S, Messina A, Mazzucco A.
1983;1:783–789. Coronary ostial enlargement to prevent stenosis
w8x Bartels C, Leyh RG, Matthias Bechtel JF, Joubert- after prosthetic aortic valve replacement. Ann
Hubner E, Sievers HH. Discrepancies between Thorac Surg 2004;77:1854–1856.
sizer and valve dimensions: implications for small w15x Ovrum E, Tangen G. Acute leaflet arrest in St.
aortic root. Ann Thorac Surg 1998;65:1631–1633. Jude Medical regent aortic valve. J Thorac
w9x Bonchek LI, Burlingame MW, Vazales BE. Cardiovasc Surg 2005;129:1446.
Accuracy of sizers for aortic valve prostheses. J w16x Grattan MT, Thulin LI. Leaflet arrest in St. Jude
Thorac Cardiovasc Surg 1987;94:632–634. Medical and CarboMedics valves: an experi-
w10x Ionescu A, Fraser AG, Butchart EG. Prevalence mental study. Eur J Cardiothorac Surg 2004;
and clinical significance of incidental para- 25:953–957.
prosthetic valvar regurgitation: a prospective w17x Walker DK, Brendzel AM, Scotten LN. The new St.
study using transoesophageal echocardiography. Jude Medical Regent mechanical heart valve:
Heart 2003;89:1316–1321. laboratory measurements of hydrodynamic per-
w11x Shapira Y, Vaturi M, Weisenberg DE, Raanani E, formance. J Heart Valve Dis 1999;8:687–696.
Sahar G, Snir E, Battler A, Vidne BA, Sagie A. w18x Vitale N, Caldarera I, Muneretto C, Sinatra R,
Impact of intraoperative transesophageal echo- Scafuri A, Di Rosa E, Contin A, Tedesco N,
cardiography in patients undergoing valve re- Pierangeli A, Abbate M, Gherli T, Casarotto D, Di
placement. Ann Thorac Surg 2004;78:579–583. Summa M, Marino B, Chiariello L, de Luca L.
w12x Astor BC, Kaczmarek RG, Hefflin B, Daley WR. Clinical evaluation of St. Jude Medical Hemo-
Mortality after aortic valve replacement: results dynamic Plus versus standard aortic valve pros-
from a nationally representative database. Ann theses: the Italian multicenter, prospective,
Thorac Surg 2000;70:1939–1945. randomized study. J Thorac Cardiovasc Surg
w13x Jin R, Grunkemeier GL, Starr A. Validation and 2001;122:691–698.
7You can also read