Brown Adipose Tissue-A Translational Perspective - Oxford Academic
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Endocrine Reviews, 2022, XX, 1–50
https://doi.org/10.1210/endrev/bnac015
Advance access publication 29 May 2022
Brown Adipose Tissue—A Translational Perspective
André C. Carpentier,1, Denis P. Blondin,2 François Haman,3 and Denis Richard4
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
1
Division of Endocrinology, Department of Medicine, Centre de recherche du Centre hospitalier universitaire de Sherbrooke, Université de
Sherbrooke, Sherbrooke, Quebec, J1H 5N4, Canada
2
Division of Neurology, Department of Medicine, Centre de recherche du Centre hospitalier universitaire de Sherbrooke, Université de
Sherbrooke, Sherbrooke, Quebec, J1H 5N4, Canada
3
University of Ottawa, Ottawa, Ontario, K1N 6N5, Canada; and
4
Centre de recherche de l’Institut universitaire de cardiologie et de pneumologie de Québec, Université Laval, Quebec City, Quebec, G1V 4G5, Canada
Correspondence: André C. Carpentier, MD, Division of Endocrinology, Faculty of Medicine, University of Sherbrooke, 3001, 12th Ave N, Sherbrooke, Quebec,
J1H 5N4, Canada. Email: Andre.Carpentier@USherbrooke.ca.
Abstract
Brown adipose tissue (BAT) displays the unique capacity to generate heat through uncoupled oxidative phosphorylation that makes it a very
attractive therapeutic target for cardiometabolic diseases. Here, we review BAT cellular metabolism, its regulation by the central nervous and
endocrine systems and circulating metabolites, the plausible roles of this tissue in human thermoregulation, energy balance, and cardiometabolic
disorders, and the current knowledge on its pharmacological stimulation in humans. The current definition and measurement of BAT in human
studies relies almost exclusively on BAT glucose uptake from positron emission tomography with 18F-fluorodeoxiglucose, which can be dissoci-
ated from BAT thermogenic activity, as for example in insulin-resistant states. The most important energy substrate for BAT thermogenesis
is its intracellular fatty acid content mobilized from sympathetic stimulation of intracellular triglyceride lipolysis. This lipolytic BAT response is
intertwined with that of white adipose (WAT) and other metabolic tissues, and cannot be independently stimulated with the drugs tested thus
far. BAT is an interesting and biologically plausible target that has yet to be fully and selectively activated to increase the body’s thermogenic
response and shift energy balance. The field of human BAT research is in need of methods able to directly, specifically, and reliably measure BAT
thermogenic capacity while also tracking the related thermogenic responses in WAT and other tissues. Until this is achieved, uncertainty will
remain about the role played by this fascinating tissue in human cardiometabolic diseases.
Graphical Abstract
Key Words: brown adipose tissue, thermogenesis, adipose tissues, obesity, insulin resistance, diabetes, glucose metabolism, lipid metabolism, energy
metabolism
Received: 28 February 2022. Editorial Decision: 25 May 2022. Corrected and Typeset: 20 July 2022
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (https://
creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the
original work is not altered or transformed in any way, and that the work is properly cited. For commercial re-use, please contact journals.permissions@
oup.com2 Endocrine Reviews, 2022, Vol. XX, No. XX
Abbreviations: 12,13-diHOME, 12,13-dihydroxy-9Z-octadecenoic acid; A2A, adenosine receptor 2A; ACTH, adrenocorticotropin; ADK, adenosine kinase; ADP,
adenosine 5′-diphosphate; ADR, adrenergic receptor; AgRP, agouti-related peptide; AMPK, adenosine 5′-monophosphate-activated protein kinase; ANGPTL4,
angiopoietin-like 4; ARC, arcuate nucleus; AT, adipose tissue; ATGL, adipose tissue triglyceride lipase; ATP, adenosine 5′-triphosphate; BAT, brown adipose
tissue; BMI, body mass index; BMP4, bone morphogenic protein 4; BMP7, bone morphogenic protein 7; BMP8b, bone morphogenic protein 8b; BMS, brain
melanocortin system; cAMP, cyclic adenosine 3’,5’-monophosphate; C/EBPs, CCAAT/enhancer proteins; ChERBP, carbohydrate-response element-binding
protein; CIDEA, cell death activator; 11C-mHED, 11C-metahydroxyephedrine; CNS, central nervous system; CPT1b, carnitine palmitoyltransferase 1b; CPT2,
carnitine palmitoyltransferase 2; CT, computed tomography; DAG, diacylglycerol; DGAT, diacylglycerol acyltransferase; DH, dorsal horn; DHA, docosahexaenoic
acid; DMH/DHyA, dorsomedial hypothalamus, dorsomedial hypothalamus/dorsal hypothalamic area; EBF2, Early B-cell factor-2; EE, energy expenditure;
ELOVL3, elongation of very long chain fatty acids protein 3; En1, homeobox protein engrailed 1; EPA, eicosapentaenoic acid; EPDR1, ependymin-related protein
1; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; FABP4, fatty acid binding protein 4; FA-CoA, fatty acyl-coenzyme A; 18FDG, 18F-fluoro
deoxyglucose; FGF21, fibroblast growth factor 21; FoxO1, forkhead box protein O1; FSF, fat signal fraction; 18FTHA, 18F-fluoro-thia-heptadecanoic acid; GABA,
γ-aminobutyric acid; GDF3, growth differentiation factor 3; GDP, guanine nucleotide diphosphate; GIP, glucose-dependent insulinotropic polypeptide; GLP1,
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
glucagon-like peptide 1; GPR, G protein–coupled receptor; HDAC, histone deacetylase; HFD, high-fat diet; iBAT, interscapular brown adipose tissue; IS, insulin
sensitivity; IL-6, interleukin 6; IML, intermediolateral; ingWAT, inguinal white adipose tissue; IR, insulin resistance; IV, intravenous; iWAT, inguinal white adipose
tissue; KO, knockout; LH, lateral hypothalamus; LPB, lateral parabrachial nucleus; LPL, lipoprotein lipase; 2-MAG, 2-monoacylglycerol; MAOA, monoamine oxi-
dase; MAPK, mitogen-activated protein kinase; MCR3, melanocortin receptor 3; MCR4, melanocortin receptor 4; miRNA, microRNA; MRI, magnetic resonance
imaging; mRNA, messenger RNA; MRS, magnetic resonance spectroscopy; mTOR, mammalian target of rapamycin; Myf5, myogenic factor 5; NAD+/NADH,
nicotinamide adenine dinucleotide, oxidized/reduced form; NDPK, nucleoside diphosphate kinase; NEFA, nonesterified fatty acids; NF-κB, nuclear factor κB;
NOD, nucleotide-oligomerization domain-containing proteins; NPY, neuropeptide Y; NST, nonshivering thermogenesis; NTS, nucleus tractus solitarius; P2X,
purinergic receptor 2X; P2Y, purinergic receptor 2Y; p38 MAPK, phospho-38 mitogen-activated protein kinase; Pax3, paired box protein 3; Pax7, paired box
protein 7; PDE3E, phosphodiesterase 3B; PEPCK, phosphoenolpyruvate carboxykinase; PET, positron emission tomography; PDGFRA, platelet-derived growth
factor receptor A; PGC1A, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A;
PKC, protein kinase C; POA, preoptic area; POMC, proopiomelanocortin; PPAR-γ, peroxisome proliferator–activated receptor gamma; PRDM16, PRD1-BF1-RIZ1
homologous domain-containing 16; PRV, pseudorabies virus; Prx1, paired-related homeobox transcription factor 1; PVH, paraventricular hypothalamus; ROS,
reactive oxygen species; RPa, raphe pallidus; Sim-1, single-minded homolog 1; SIRT1, sirtuin 1; SIRT5, sirtuin 5; SNS, sympathetic nervous system; T3, 3,5,3′-tri-
iodothyronine; TEE, total energy expenditure; TMEM26, transmembrane protein 26; TNAP, tissue-nonspecific alkaline phosphatase; TRPV1, transient receptor
potential cation channel subfamily V member 1; TSPO, translocator protein; WAT, white adipose tissue; T2D, type 2 diabetes; TCA, tricarboxylic acid cycle;
TGs, triglycerides; TGF, transforming growth factor; TGR5, G protein–coupled bile acid receptor Gpbar1; TLR, toll-like receptor; TNF-α, tumor necrosis factor
alpha; TRIB1, tribbles pseudokinase 1; TRL, triglyceride-rich lipoprotein; TRPM8, transient receptor potential melastatin 8; TRPV1, transient receptor potential
vanilloide 1; TSH, thyrotropin; UCP1, uncoupling protein 1; VEGFA, vascular endothelial growth factor A; VLDL, very low-density lipoprotein; VMH, ventromedial
hypothalamus.
Brown adipose tissue (BAT) displays the unique capacity
ESSENTIAL POINTS to generate heat through uncoupled oxidative phosphor-
• The current standard definition of brown adipose ylation. Its thermogenic potential confers on small mam-
tissue (BAT) in humans is based on glucose metab- mals, in which it is relatively abundant, the ability to
olism measured with 18F-fluoro-deoxyglucose–posi- survive in the cold without relying on shivering to gen-
tron emission tomography/computed tomography erate heat. This outstanding thermogenic property makes
(18FDG-PET/CT). BAT a very attractive therapeutic target for obesity and its
• BAT is a thermogenic organ that is effectively re- cardiometabolic complications, although its presence in hu-
cruited on acute and chronic cold exposure. mans has been contested for years. First described in mar-
• BAT primary source of energy for thermogenesis is mots by Gessner in 1551 (1) and identified early by Aherne
its own triglyceride (TG) content, with glucose and and Hull in newborn infants (2) and then by Heaton in
amino acids contributing to rapid intracellular TG human necropsies (3), metabolically active BAT was dem-
repletion. onstrated in vivo in adults in 2003 through positron emis-
• The activation of BAT thermogenesis is primarily sion tomography (PET) with the glucose analogue tracer
from sympathetic nervous system (SNS) outflow acti-
18
F-fluorodeoxiglucose (18FDG) (4, 5). Three back-to-back
vated by cold exposure; modulation of BAT thermo- papers in The New England Journal of Medicine in 2009
genesis by local metabolites and systemic hormones (6–8) then fanned the flames of BAT investigation not only
such as glucocorticoids, thyroid, and sex hormones is in humans, but also in preclinical models. Since this evi-
possible, but still unascertained in humans. dence for metabolically active BAT in adult human was
• BAT glucose uptake is often reduced in conditions as- published, there has been an exponential accumulation of
sociated with insulin resistance, without a concomi- knowledge on the possible physiological and pathophysio-
tant reduction of BAT thermogenic activity. logical roles of this fascinating tissue.
• Because BAT mass in vivo is currently defined from We offer herein a review of the current state of knowledge
glucose uptake, BAT thermogenic capacity is likely on BAT, focusing on investigations in humans while offering
underestimated in insulin-resistant states and its con- a translational perspective on the pathophysiological roles of
tribution to energy expenditure awaits new methods BAT, beige, and white adipose tissues (WAT) as an integrated
allowing more specific quantification. thermogenic organ. We first overview the brown adipocyte
• No drug tested thus far selectively activates BAT in cellular metabolism and then discuss the current functional
humans; all drugs tested also activate white adipose definition of BAT and the tools employed to this effect. We
tissue and/or cardiovascular responses that also con- review the control of BAT by the nervous and endocrine
tribute to whole-body energy expenditure. systems and local and circulating metabolites. A discussion
on the plausible roles of BAT in human physiology, energyEndocrine Reviews, 2022, Vol. XX, No. XX 3
balance, and in cardiometabolic disorders follows. We finish histone deacetylases, are also involved in the regulation of
with a review of the attempts at pharmacological activation brown adipocyte thermogenesis (39, 40). SIRT1 increases
of BAT in humans and by offering our perspective on gaps the expression of PRDM16 and brown adipocyte differenti-
and future directions of clinical BAT investigation. ation through deacetylation (40). SIRT1 furthermore reduces
brown adipocyte apoptosis and endoplasmic reticulum (ER)
stress during high-fat diet (HFD) conditions in mice (41). In
Cellular Biology of Brown Adipose Tissue addition, several microRNAs (miRNAs) are produced by
Transcriptional and Epigenetic Regulation of white and brown adipocytes (42), and some have been shown
Thermogenic Adipocytes to inhibit or stimulate brown adipocyte differentiation (sum-
Thermogenic, or brown adipocytes, display a typical mo- marized in Alcalá et al) (43), further supporting epigenetic
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
lecular signature, including relatively high expression levels of regulation of brown fat recruitment. Epigenetic mechanisms
uncoupling protein 1 (UCP1), cell death activator (CIDEA), have also been evoked in the regulation of the sympathetic
and peroxisome proliferator–activated receptor gamma output signal driving BAT thermogenesis. For example,
coactivator 1-alpha (PGC1A) in PRD1-BF1-RIZ1 homolo- hypothalamic miR-33, induced by ER stress, is implicated in
gous domain–containing 16 (PRDM16) positive cells (9–12), increasing the sympathetic tone necessary for cold- and high-
and histopathological features, including multiple small lipid fat diet-induced thermogenesis in mice, through the reduction
vacuoles and rich mitochondrial content (3, 13–16), easily of γ-aminobutyric acid (GABA) A receptor–related gene ex-
distinguishable from WAT. Thermogenic adipocytes develop pression (44).
from heterogeneous stem cells of mesodermal origin that The characterization of the heterogeneous origins and the
variously express homeobox protein engrailed 1 (En1), myo- transcriptional and posttranscriptional control mechanisms of
genic factor 5 (Myf5), paired box protein 7 (Pax7), Pax3, and brown adipocyte development and thermogenic programing
paired-related homeobox transcription factor 1 (Prx1) (17). is of outstanding importance for the development of future
Peroxisome proliferator–activated receptor gamma (PPAR-γ) therapeutic avenues to exploit the unique BAT thermogenic
and CCAAT/enhancer proteins (C/EBPs) are necessary, but properties. Readers are referred to more complete recent re-
not sufficient for brown adipocyte differentiation (18–20). views on this important topic (45–47). At the moment, how-
Early B-cell factor-2 (EBF2) recruits PPAR-γ to selective gene- ever, this knowledge is not directly applicable for the in vivo
binding sites that promote brown adipogenesis and thermo- characterization or modulation of BAT function in humans.
genic programming of myoblasts and preadipocytes (21).
PRDM16 is essential in Myf5+ cell commitment to brown Mitochondrial Function and Energy Uncoupling
adipogenesis (22, 23) and thermogenesis programming of The mitochondria of classical brown adipocytes have the
adipocytes from this lineage (20, 24, 25). Its absence in mice unique capacity for large inner membrane proton conductance
does not however compromise classical BAT early develop- that diffuses the proton gradient independent from adenosine
ment, but does impair WAT browning (ie, beige adipocytes) 5′-triphosphate (ATP) production, leading to heat produc-
and thermogenic activity on chronic cold exposure (26) and tion as the main product of the mitochondrial respiration of
impairs the maintenance of an effective interscapular BAT these cells. The presence and activation of UCP1, described
(iBAT) thermogenic phenotype throughout the life of the more than 40 years ago (48), is responsible for this unique
mouse (27). Indeed, thermogenic adipocytes distinct from the feature. There is still debate about the exact mechanism by
classic brown adipocyte (ie, PRDM16 negative) have been which UCP1 exerts this profound mitochondrial uncoupling
identified (28). Bone morphogenic protein 7 (BMP7) is an- (49–51), but the basic function and regulation of UCP1 is felt
other critical factor for brown adipogenesis and thermogenic to be the same between rodent models and humans. UCP1 is
programing (29). In addition to these prothermogenic tran- downregulated by ATP or other purine nucleotides binding
scriptional regulators, transcriptional brakes have been de- (52, 53), and UCP1 binding to radiolabeled guanine nucleo-
scribed (30) such as zinc finger protein 423 expressed by WAT tide diphosphate (GDP) has been used as a functional marker
that prevent the conversion of white adipocyte precursors to of UCP1 activation in vitro (54, 55). “Unmasking” of these
thermogenic adipocytes (31–33). GDP binding sites occurs in BAT mitochondria after adren-
Epigenetic modifications such as DNA methylation are ergic or cold stimulation, independent of change in UCP1
important regulators of adipose tissue function (34). DNA protein expression (54, 55). A direct competition for purine
demethylation has generally been associated with stimula- nucleotide binding sites by long-chain fatty acids generated
tion of the adipocyte thermogenic phenotype. For example, by intracellular triglyceride (TG) lipolysis, leading to induc-
alpha-ketoglutarate–stimulated demethylation under the tion of a protonophoric conformation of UCP1, has been
control of adenosine 5′-monophosphate (AMP)-activated proposed (56). The association between long-chain fatty acids
protein kinase (AMPK) increases PRDM16 expression and with UCP1 may alternatively form protonable carboxylate
brown adipogenesis and thermogenesis (35). Lysine-specific groups in the mitochondrial matrix (57). Another possibility,
demethylase-1 promotes brown adipocyte thermogenesis by the protonophoretic model, stipulates UCP1-independent
repressing adipose tissue hydroxysteroid 11-β-dehydrogenase intramitochondrial transport of protonated long-chain fatty
isozyme 1 and therefore reducing local corticosterone levels acids, followed by deprotonation in the mitochondrial matrix
(36). Another histone, H3 lysine 9 demethylase, JMJD1A, and UCP1-dependent export or long-chain fatty acids (58,
upregulates β-adrenergic receptor and PPAR-γ and stimu- 59). Finally, the shuttling model suggests simultaneous trans-
lates adipose browning (37). Histone deacetylation also regu- port of a long-chain fatty acid and a proton with the inability
lates adipocyte thermogenesis. In mice, histone deacetylase of UCP1 to release the long-chain fatty acid (60). Using
11 (HDAC11) suppresses iBAT and iWAT thermogenic magnetic nuclear resonance and functional mutagenesis, the
programming and adaptive thermogenesis during cold ex- binding of a long-chain fatty acid to UCP1 was shown to be
posure (38). Sirtuins (eg, SIRT1, SIRT5), NAD+-dependent necessary for UCP1-mediated proton flux (61). Whatever the4 Endocrine Reviews, 2022, Vol. XX, No. XX
precise molecular mechanism, long-chain fatty acids are thus production is thus necessary to sustain lipogenesis in adipo-
generally considered to be the most likely activation signal cytes (78). Glycerol-3-phosphate synthesis from glycolysis and
of UCP1. glyceroneogenesis, and esterification reactions needed for TG
Intracellular TG lipolysis in brown adipocytes is the likely synthesis, are also ATP-requiring processes (77). The in vivo
source of long-chain fatty acids for the activation of UCP1- energy cost of TG deposition is 1.5 to 2 times higher when
mediated thermogenesis. This was supported in vivo by the both fatty acids and glucose are available compared to fatty
demonstration of the inhibition of BAT thermogenesis using acids alone (79). Because very rapid rates of TG synthesis and
nicotinic acid–mediated suppression of intracellular lipolysis glucose uptake occur in thermogenically active BAT (80), im-
in rats and humans (62, 63). However, BAT-specific knockout portant utilization of ATP is thus expected in brown adipocytes.
(KO) of adipose tissue TG lipase (ATGL) or its activating It is therefore highly likely that a substantial fraction of
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
protein CGI-58 demonstrated that BAT metabolic activity the thermogenic adipocyte energy expenditure is driven by
can also be fueled by nonesterifed fatty acids (NEFAs) from UCP1-independent processes. Recently, single-nuclei RNA-
WAT lipolysis or from intravascular lipolysis of TG-rich sequencing analyses of mouse WAT revealed heterogeneity of
lipoproteins (TRLs) (64, 65). It is also possible that another thermogenic adipocytes in response to cold or beta-3 adren-
downstream metabolite of intracellular lipolysis or norepin- ergic stimulation: One population displays the classic beige
ephrine signaling in BAT may activate UCP1-mediated un- thermogenic program with increased mitochondrial oxidative
coupled respiration (51). For example, mice with adipose genes, whereas another is characterized by increased expres-
tissue–specific KO of adipose alpha/beta-hydrolase domain sion of genes involved in NEFA/TG cycling (81). Intercellular
6, normally hydrolyse 2-monoacylglycerol (2-MAG), display exchange of energy metabolites between adipocytes within
greater cold tolerance, enhanced WAT NEFA/TG cycling, and BAT is therefore likely (discussed in subsequent sections).
iBAT thermogenesis on cold exposure through 2-MAG–me- In summary (Fig. 1), UCP1-mediated mitochondrial un-
diated activation of PPAR-α compared to control mice (66). coupling is the primary driver of the remarkable thermogenic
Mitochondrial reactive oxygen species (ROS) during res- activity of BAT in rodents and humans. While a phosphocrea-
piration also contributes to UCP1 activation in the brown tine/creatine futile cycle may contribute to further reduction
adipocyte (67). Other lipids such as peroxisome-derived of the ATP/ADP ratio of brown adipocytes, ATP production
plasmalogens (ether phospholipids) may also be important is nevertheless essential to sustain BAT TG synthesis that
for cold-induced adipose mitochondrial mass and thermo- in turn constitutes the primary source of fatty acids driving
genesis (68). UCP1-mediated thermogenesis. A high rate of intracellular
Early studies by Golozoubova and colleagues (69) showed NEFA/TG cycling is an important energy-requiring process
that only shivering thermogenesis could compensate the ab- that characterizes this tissue. It would be very interesting to
sence of UCP1 for cold-induced thermogenesis. However, a apply imaging methods able to tract changes in cellular ATP
series of alternative nonshivering thermogenic mechanisms production (eg, 31P-magnetic resonance spectroscopy [MRS])
were proposed following the demonstration that UCP1 KO and thermogenesis (11C-acetate or 15O-O2 PET) to simultan-
mice gradually adapted to cold display–increased iWAT en- eously track these metabolic rates and determine the relative
ergy expenditure (70), a mechanism dependent on the pres- contribution of uncoupled vs coupled respiration in activated
ence of leptin (71). Creatine has been shown to drive a futile human BAT. To the best of our knowledge, no group has per-
cycle leading to energy expenditure in beige and brown adi- formed such a study thus far.
pocytes able to compensate for the absence of UCP1 in mice
(72). Creatine availability and transport to adipocytes is key Energy Substrate Metabolism
to this thermogenic mechanism (73). However, creatine sup- As discussed in a subsequent section, glucose metabolism has
plementation failed to increase cold-induced thermogenesis been the first and still is the main in vivo process by which BAT
and BAT 18FDG accumulation and volume of activity in vivo is identified and defined in vivo. BAT glucose uptake is stimu-
in healthy individuals on a diet characterized by low creatine lated by norepinephrine and occurs concurrently with acti-
intake (a vegan diet) (74). More studies are needed to under- vation of BAT thermogenesis. We have extensively reviewed
stand the implication of cellular creatine availability for BAT BAT glucose metabolism in previous works (80, 82). The
thermogenesis in vivo in humans. major conclusions of these reviews are still currently relevant.
Creatine kinase B, which is activated by cAMP in brown From a pathophysiological standpoint, 2 key points must be
adipocytes, activates a creatine-mediated futile cycle liber- emphasized. First, glucose is mostly used for lactate and TG
ating very large amounts of adenosine 5′-diphosphate (ADP) synthesis in BAT (83–85), not to drive thermogenesis. Glucose
to accelerate basal and β-adrenergic–stimulated cellular res- contributes to the synthesis of fatty acids esterified into TGs
piration in a UCP1-independent fashion; the absence of that are simultaneously hydrolyzed to drive brown adipocyte
creatine kinase B in BAT or in all adipose tissues leads to thermogenesis (86). Glucose availability increases insulin-
increased weight gain and higher glucose levels in mice (75). mediated TG synthesis and fatty acid storage in adipocytes in
The protein catalyzing phosphocreatinine hydrolysis pro- vitro (87–89), but also in vivo (90). Carbohydrate-response
cess in BAT mitochondria was recently shown to be tissue- element-binding protein (ChERBP), which is activated by
nonspecific alkaline phosphatase (TNAP), which is potently cold exposure and by increased glucose availability (91), and
induced by cold exposure (76). Genetic ablation of TNAP in which controls the genes that drive de novo lipogenesis, plays
adipocytes leads to reduced whole-body resting energy ex- an important role in BAT TG accumulation in mice (92). Rats
penditure and obesity in mice (76). adapted to a carbohydrate-poor, protein-rich diet display re-
One important ATP-requiring process in thermogenically duced BAT de novo lipogenesis (93, 94). The number of acyl-
activated brown adipocytes is lipogenesis, which theoretic- chain double bonds and methylene-interrupted double bonds
ally uses 24% of the energy generated from the metabolism is lower in BAT vs WAT, suggesting higher levels of saturated
of the glucose molecules to drive this process (77). ATP fatty acids from de novo lipogenesis in BAT (95). Therefore,Endocrine Reviews, 2022, Vol. XX, No. XX 5
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
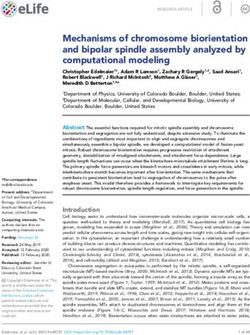
Figure 1. Brown adipocyte energy metabolism. Long-chain fatty acids (FA-CoA) activate uncoupling protein 1 (UCP1) and are the major energy
source of the brown adipocyte thermogenesis. The main source of these FA-CoA is intracellular triglyceride (TG) lipolysis, but circulating nonesterified
fatty acids (NEFA) and triglyceride-rich lipoproteins (TRL), through lipoprotein lipase (LPL)-mediated lipolysis, also contribute fatty acids to drive
thermogenesis. Glucose, branched-chain amino acids (BCAA), glutamate, and other sources of energy contribute mainly to drive anaplerosis and
cataplerotic processes such as de novo lipogenesis (DNL) and glycerol synthesis that are essential to replete intracellular triglycerides and to sustain
the very high rate of TG/nonesterified fatty acid cycling necessary for brown adipose thermogenesis. In addition to UCP1, a phosphocreatine/creatine
(PCr/Cr) futile cycle contributes to reduce the ATP/ADP ratio and drive mitochondrial thermogenesis. ADP, adenosine 5′-diphosphate; ATP, adenosine
5′-triphosphate; BCAA, branched-chain amino acids; Cr, creatine; DNL, de novo lipogenesis; FA-CoA, long-chain fatty acyl coenzyme A; LPL, lipoprotein
lipase; NEFA, nonesterified fatty acids; TG, triglycerides; PCr, phosphocreatine; TCA, tricarboxylic acid cycle; TRL, triglyceride-rich lipoproteins; UCP1,
uncoupling protein 1.
glucose metabolism likely contributes to maintain BAT TG this intracellular BAT TG/NEFA cycling during acute cold
content, its primary energy supply for cold-induced thermo- exposure in humans using the combination of 11C-acetate,
genesis. Second, BAT glucose uptake is dependent of insulin, 11
C-palmitate, 18FDG-PET and magnetic resonance imaging
not only of norepinephrine-induced thermogenic activation, (MRI) methods (Clinicaltrials.gov No. NCT05092945).
and insulin resistance (IR) and low BAT glucose uptake may In rodents, fatty acids in circulation are also an important
occur without reduction in acute cold-induced BAT thermo- source of substrates to drive BAT thermogenesis. In mice, gen-
genesis (discussed in previous and subsequent sections). To the etic KO of genes essential for BAT intracellular TG lipolysis
best of our knowledge, in vivo BAT glucose metabolism under leads to upregulation of the utilization of circulating fatty
insulin vs noradrenergic stimulation has not been directly acids to drive thermogenesis (64, 65). The absence of BAT
measured in animal models or humans with methods able to intracellular lipid droplets in BAT-specific DGAT1 + DGAT2
determine oxidative vs nonoxidative glucose metabolism (ie, KO mice does not prevent BAT thermogenesis and results
11
C-glucose PET). Therefore, the metabolic fate of glucose in in increase glucose and circulating fatty acid utilization to
BAT under these 2 different stimulations is unknown. drive adaptive thermogenesis and resistance to diet-induced
Intracellular TG lipolysis is critical for the acute stimula- glucose intolerance (109). Fatty acid transport protein, fatty
tion of BAT thermogenesis in vitro (96) and in vivo in rats acid binding protein, and CD36 are expressed in brown adi-
(62) and humans (63). BAT TG content is reduced (97–107) pocytes and are important for thermogenesis (110–112). In
and BAT glycerol release is enhanced (84) during acute cold mice, activated BAT has the capacity to clear most circulating
exposure in humans. From the glycerol release, it has been TRL lipids (112–114). Lipoprotein lipase (LPL) expression is
estimated that approximately 65 nmol/g/min of NEFA are re- increased during cold exposure specifically in BAT, but not in
leased in this condition (84). This is likely an underestimation WAT in humans (115). Serum angiopoietin-like 4 (ANGPTL4),
given the presence of high levels of glycerol kinase in human which inhibits LPL activity, is increased on cold exposure in
BAT (84, 108), that allows the recycling of glycerol produced humans (116). In mice, BAT Angptl4 expression is however
during lipolysis for TG resynthesis. BAT TG content reaches reduced, whereas that of WAT is increased during cold ex-
a nadir within 35 minutes and plateaus thereafter in young posure, providing a potential mechanism to shuttle TRL TG
healthy men on cold exposure (105). This demonstrates rapid content to BAT via LPL-mediated lipolysis to provide fatty
and active TG replenishment during BAT metabolic activa- acids to drive thermogenesis (117). In addition to their TG
tion. Currently, no one has measured the rate of this BAT content, TRL particles can also be cleared by BAT in rodents
TG/NEFA cycling in vivo in humans during BAT metabolic (112–114). 18F-labeled BODIPY-TG-chylomicron-like particle
activation. Studies are ongoing in our laboratory to quantify uptake by BAT is increased in mice on acute, but not chronic,6 Endocrine Reviews, 2022, Vol. XX, No. XX
cold stimulation (118). TRL particles are most likely taken up is very rapidly activated with ongoing and rapid replenish-
by BAT endothelial cells through endocytosis; lysosomal acid ment of intracellular TG from glucose and other anaplerotic/
lipase then hydrolyses TRL-TG, which stimulates endothelial cataplerotic sources such as branched-chain amino acids.
cell beta oxidation and activates hypoxia-inducible factor-1 More studies are needed to characterize this BAT TG/NEFA
alpha-dependent proliferation of endothelial cells and adipo- cycling in vivo on metabolic activation.
cyte precursors (119).
However, most studies in humans showed no substantial
change or even increase in plasma TG levels during acute cold
In Vivo Methods to Define Brown
exposure (63, 101, 103, 120, 121). Four-week cold acclima-
Adipose Tissue
tion that increases BAT thermogenic activity up to 2.6-fold PET coupled with computed tomography (PET/CT) and MRI-
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
on acute cold exposure also does not lead to a reduction based methods have been thus far the most important modalities
in fasting (122) or even postprandial levels of total, chylo- for the in vivo investigation of BAT in humans. Thermographic
micron or very low-density lipoprotein (VLDL) TG levels and other optic methods were proposed very early to study BAT
(104). In a later study, we directly measured BAT uptake of activity in vivo (137, 138). These methods may be more inform-
dietary fatty acids, which are transported to tissues largely ative in preclinical studies because of the capacity to use fluores-
as chylomicron-TG, using the oral 18F-FTHA PET method cent labels, the much smaller size of the animals, and the more
(123). During cold exposure, BAT takes up dietary fatty acids superficial iBAT depot (typically within 5 mm of the skin sur-
at greater rates than subcutaneous WAT and resting skeletal face in rodents, whereas supraclavicular BAT in adult humans is
muscles, but at lower rates than the heart and the liver (104). > 5-10 mm deep, varying widely depending on the thickness of
BAT dietary fatty acid uptake is also unchanged in the face the subcutaneous adipose tissue depot). However, they are very
of a 2.6-fold increase in BAT oxidative metabolism after cold limited by their low penetration depth and by the nonspecific
acclimation and accounts for only 0.3% of total body utiliza- signal mixing superficial vasculature of the skin, subcutaneous
tion of dietary fatty acids. fat, and muscle in addition to any BAT-mediated signal. Other
Activated BAT uses succinate (124, 125) and branched- emerging methods such as contrast-enhanced echography have
chain amino acids (126) at high enough rates to provide a sys- been proposed, but have not been largely used in human investi-
temic metabolic sink for these metabolites in mice. BAT also gations. Excellent reviews exist of these various methods (139–
uses glutamate at greater rates during acute cold exposure 143). We restrict our discussion herein to some of the PET/CT
in humans, but at much lower rates than circulating glucose and MRI-based methods that have provided major insights into
(84). Circulating valine, an anaplerotic substrate (127), is re- BAT metabolic function.
duced during cold exposure in humans, but only in individ- From an integrative physiology and clinical point of view,
uals displaying positive BAT 18FDG uptake (126). However, BAT is best defined by its function as a thermogenic adipose
skeletal muscles are the most important site for branched- tissue (80). It is the revelation of highly metabolically active
chain amino acid metabolism in mice and humans (128). adipose tissues by 18FDG PET/CT that convinced the broad
Therefore, metabolic activation of skeletal muscles is a much scientific community of the existence and possible physio-
more likely explanation for cold-induced changes in circu- logical relevance of BAT in adult humans (6–8, 144–146). The
lating branched-chain amino acid levels in humans (129). mere presence of molecular and histopathological features of
One possible metabolic fate of branched-chain amino adipose tissue browning however does not necessarily trans-
acids (valine, isoleucine), glutamate, and succinate is the late into in vivo significant thermogenic capacity, as shown by
anaplerotic/cataplerotic pathways, that is, glyceroneogenesis the absence of detectable in vivo thermogenic activity despite
and de novo lipogenesis (127). Glyceroneogenesis (ie, the robust browning of WAT after prolonged cold exposure in ro-
production of glycerol-3-phosphate from pyruvate, lac- dents (147, 148). The molecular signature of supraclavicular
tate, and amino acids) is essential for TG synthesis in mice BAT depots in humans is more similar to that of “beige” than
(130). The absence of PEPCK, the rate-limiting enzyme for brown adipocytes of rodents (149). Ex vivo mitochondrial
glyceroneogenesis, causes a marked reduction in WAT and respiration of brown adipocytes appears increased in mice ac-
BAT TG content (83, 131). Glyceroneogenesis is stimulated climated at room temperature vs in humans (150). Despite
in adipocytes by norepinephrine and by PPAR-γ stimulation these differences, Ucp1 content is similar between human
(130, 132–135). It is also activated in vivo in rats by cold ex- and mouse BAT (150) and the typical supraclavicular adipose
position (136) even on a carbohydrate-free, protein-rich diet tissue depots in humans display increased in vivo thermogenic
(94), suggesting that amino acids can substitute for carbohy- activity on acute cold exposure (101), as does the iBAT in ro-
drates to sustain BAT TG synthesis. UCP-1–driven KO of the dents (147, 148).
branched-chain amino acids transporter SCL25A44 results in There have been attempts at developing noninvasive
considerable reduction in BAT thermogenesis (126). However, methods to identify BAT using PET tracers targeting spe-
in mice exposed to mild cold, branched-chain amino acids cific molecular ligands (151) or outer mitochondrial mem-
account for only approximately 6% of Krebs cycle carbon brane proteins (ie, translocator protein [TSPO]) (152–155)
flux (128) and are therefore not important contributors for instead of its metabolic function. However, with the excep-
BAT cataplerotic pathways. tion of one small human study (n = 3 participants) that re-
In summary (see Fig. 1), fatty acids are the most important ported relatively selective 11C-PBR28 (a TSPO radioligand)
energy substrate to drive BAT thermogenesis. Its source is uptake in BAT vs WAT at room temperature (155), no such
primarily the brown adipocytes’ TG content on acute acti- method has been used in humans. MRI with fat fraction and/
vation, although circulating NEFA and TG may likely in- or T2* mapping to quantify mitochondrial content from the
creasingly contribute to thermogenesis with sustained BAT signal of iron-containing heme has also been used, but with
activation. Intracellular brown adipocyte TG/NEFA cycling limited capacity to differentiate BAT from WAT (reviewed inEndocrine Reviews, 2022, Vol. XX, No. XX 7
[139–143]). We therefore favor the definition of BAT as an the addition of another method to measure tissue intersti-
adipose tissue displaying substantial thermogenesis in vivo. tial volume (eg, contrast-enhanced MRI) is necessary for
The next question is how best to detect and measure this the optimal precision of 11C-acetate PET determination of
thermogenic adipose tissue. Currently, the combination of BAT oxidative metabolism (183). Unless one has access to
high glucose uptake using intravenous (IV) 18FDG adminis- a PET scanner with an extended field of view including the
tration with PET in a tissue displaying the anatomical charac- neck, thorax, and abdomen (184), these methods are also not
teristics and high fat content compatible with adipose tissue, amenable to image all of the BAT-containing fat depots and
determined by CT or MRI, is still the standard definition of therefore cannot measure the entire BAT volume and thermo-
BAT (80, 156). This glucose-using adipose tissue is scattered genic capacity. The very short radioactive half-lives of these
in multiple small depots in the supraclavicular, paravertebral, tracers also necessitate a cyclotron and radiochemistry facil-
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
pericardial, and suprarenal regions (157). Measured using ities at the site of scanning, making these methods inapplic-
this method, BAT volume in human adults spans 2 orders of able widely. Finally, 15O and 11C produce lower-resolution
magnitude from a few to hundreds of milliliters (82). This PET images than 18F (full width of half maximum of 2.48,
huge variability depends largely on environmental exposure 0.92, and 0.54 mm, respectively) (185), primarily because of
to cold (102, 158–160), but also on biological factors such as the energy of their emitted positrons, which determines their
age, sex, visceral adiposity, IR/diabetes, cardiometabolic risk, diffusion range.
and circadian rhythm (8, 146, 157, 161–169) and the use of Alternative methods have been proposed to assess BAT in
some drugs (eg, β-adrenergic agonists and antagonists) (121, vivo in humans. Imaging of sympathetic nerve activity by ex-
170–172). ploiting the presynaptic reuptake of norepinephrine demon-
Critically, BAT glucose uptake is not a measure of thermo- strated by the 1970 Nobel Prize in Physiology or Medicine
genesis. First, a large fraction of BAT glucose uptake is me- winner Ulf von Euler is very attractive and has been at-
tabolized into lactate or glycerol ex vivo (83), which was tempted with different PET tracers including 11C-epinephrine,
confirmed in vivo during acute cold exposure in humans (84). 18
F-fluoro-norepinephrine, 11
C-metahydroxyephedrine
Second, glucose uptake in BAT does not need the activation ( C-mHED),
11 18
F-fluoro-propoxy-benzylguanidine, and
of thermogenesis in rodents (173, 174) and can be dissociated 18
F-fluoro-dopamine (186–189). Sympathetic activation is the
from in vivo thermogenic activity in humans (103). Third, in- endogenous driver of BAT thermogenesis, but is not neces-
sulin administration increases BAT glucose uptake, but not sarily directly proportionally to the ensuing thermogenic re-
blood flow, suggesting dissociation between glucose uptake sponse. Furthermore, this method would not allow detection
and thermogenesis under varying degree of insulin stimula- of the BAT thermogenic response to exogenous stimulation
tion (175). BAT glucose uptake is reduced in genetic variants (ie, β-adrenergic agonists).
of IR (176) and in conditions that induce IR such as pro- The most promising current approach to measure total
longed fasting (177), glucocorticoid treatment (178), chronic thermogenic adipose tissue mass is through the detection of
ephedrine administration (179), and fructose overfeeding the fat fraction shift that occurs during activation of BAT
(Richard, Blondin, Carpentier et al. Unpublished). In the thermogenesis. BAT TG content is hydrolyzed and mobil-
latter randomized, controlled, crossover study, 2-week high- ized within 1 to 3 hours through sympathetically stimulated
fructose, but not high-glucose, feeding led to a significant intracellular lipolysis. This response can be seen using CT
reduction of cold-induced BAT glucose uptake without a radiodensity or the MRI Dixon method or proton MRS that
change in thermogenesis and before any significant change demonstrates a shift of BAT water-to-fat ratio, not observed
in systemic IR. This demonstrates the exquisite sensitivity of in WAT or in shivering muscles (97–107). Disappearance of
BAT glucose uptake to dietary and potentially other lifestyle intracellular BAT TG, as opposed to glucose uptake, is not
changes, drugs, and health conditions leading to deterioration necessarily affected by age and type 2 diabetes (T2D) status
of cardiometabolic health, without necessarily altering BAT at equivalent cold exposure (103). In vivo inhibition of intra-
thermogenic capacity. cellular TG lipolysis using nicotinic acid suppresses this BAT
BAT thermogenesis can be assessed directly using the shift of water-to-fat ratio and inhibits BAT thermogenesis
15
O-O2 (180, 181) or the 11C-acetate (101) PET methods. in rats and humans (62, 63). Three-dimensional mapping of
We used the 11C-acetate combined with 18FDG PET method this shift is possible using the MRI Dixon method (100, 190,
for 3-dimensional mapping of supraclavicular BAT thermo- 191). In healthy young men during acute cold exposure, the
genic activity in vivo and found a large degree of heterogen- supraclavicular BAT fat fraction declines in voxels displaying
eity of response to acute cold stimulation vs 18FDG activity. 60% to 100% fat fraction at baseline, whereas it increases in
Unfortunately, these methods rely on PET dynamic scan- voxels displaying below 30% fat fraction (192). Therefore, a
ning as the tissue metabolism of oxygen and acetate is very cold-induced shift in BAT water-to-fat ratio measured with
fast. Although 11C-acetate can be modeled relatively simply this method is quite heterogeneous. Furthermore, in vitro ex-
using monoexponential function fitting of the rapid BAT periments have shown that up to 50% of fatty acids hydro-
tissue signal decline to assess oxidative metabolism and lyzed by BAT could be released in the extracellular media
tissue peak activity to assess blood flow (101), this method (193) and subsequently oxidized or reesterified elsewhere.
does not assess nonoxidative metabolism of 11C-acetate. Therefore, more studies are needed to understand in situ BAT
Multicompartmental modeling of 11C-acetate (182) offers TG and fatty acid metabolism to interpret appropriately the
more specific assessment of these parameters and has the dynamic BAT changes in water-to-fat ratio.
added advantage of assessing acetate retention into tissue me- A promising and very versatile MRI-based modality for
tabolism (ie, anaplerotic/cataplerotic pathways) (127). More BAT imaging is deuterium metabolic imaging. This MRS tech-
studies using this novel method are needed to determine BAT nique allows the noninvasive imaging of tissue metabolism of
nonoxidative metabolism of 11C-acetate in humans. Ideally, deuterium-labeled tracers to study rapidly proliferative cells8 Endocrine Reviews, 2022, Vol. XX, No. XX
(194), hormone (195), or energy substrate metabolism (196). of most of the current human data on BAT, as will be dis-
A clear added value of this method over that offered by PET is cussed in the next sections.
the possibility of specifically tracing the appearance of down-
stream metabolites (eg, deuterated lactate and glutamate
from deuterated glucose) in tissues. BAT glucose uptake and Regulation of Brown Adipose Tissue Activity
metabolism into lactate and glutamate was reported in cold- and Capacity
acclimatized rats in one study using deuterium metabolic im- Central Nervous System Regulation of Brown
aging, showing promising results (197). One human study is Adipose Tissue Activity and Capacity
ongoing (Clinicaltrials.gov No. NCT04060745) using this Two models have been proposed to describe the homeostatic
modality after oral ingestion of deuterated glucose. Because control of body temperature: a feed-forward or feedback
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
deuterium-labeled fatty acids are available and safe for use model. The first refers to a preemptive increase in thermogen-
in humans, this method appears very promising for future in esis in response to skin cooling, resulting in the stimulation
vivo investigations of BAT fatty acid uptake and esterification of thermoeffectors before any changes in body temperature
into TG. occurs (198). The second model refers to body temperature
There is clearly a gap between the in vivo definition of being regulated by “independent thermoeffector loops, each
BAT, resting solely on imaging, and the presence of BAT using having its own afferent and efferent branches,” with each
histopathological methods. Because BAT thermogenesis is the thermoeffector being triggered by a unique combination of
feature that defines BAT in vivo, BAT thermogenesis has to superficial (skin) and deep (core) temperature (199). Although
be activated during imaging, that is, some stimulus needs to BAT appears to express temperature-sensitive receptors, they
be applied. In turn, this stimulus needs to be controlled to be likely modulate the thermogenic activity rather than serve as
able to compare experimental groups or treatments. In our the primary triggering signal.
opinion, standardized cold stimulus applied to a large pro- BAT thermogenic capacity (brown cell differentiation, pro-
portion of the body surface area that results in the same rate liferation, mitochondrial biogenesis, and increased UCP1
of heat loss (ie, same differential temperature in and out of protein content) and thermogenic activity (uncoupling ac-
the cold-water perfusion system) and therefore the same in- tivity and activation of intracellular TG lipolysis) are under
crease in whole-body energy expenditure is the best condition a dynamic brain control of BAT’s sympathetic output innerv-
to measure BAT thermogenic response in humans. ation (200–204). Different sympathetic activating stimuli
In summary, the current standard definition of BAT still lead to specific patterns of fat depot stimulation, with cold
rests on 18FDG-PET/CT, as no other method has yet gained predominantly stimulating BAT and WAT (205) and hypo-
wide recognition and applicability in humans (Fig. 2). This glycemia predominantly stimulating WAT (206). It is note-
definition is based on BAT glucose metabolism, not thermo- worthy that the brain control of BAT and WAT has essentially
genesis. This has profound implications for the interpretation been studied in laboratory rodents, mainly in rats, hamsters,
Figure 2. Definition of brown adipose tissue through metabolic imaging. First, computed tomography (CT) or magnetic resonance imaging (MRI)
is necessary for anatomic definition and quantification of tissue fat content. Second, metabolic function of the fat tissue needs to be measured.
The standard procedure for the latter is positron emission tomography (PET) with intravenous administration of 18F-fluoro-deoxyglucose (18FDG) that
measures glucose uptake. Other experimental approaches can provide measurement of other important characteristics of brown adipose tissue
such as oxygen utilization or carbon dioxide production (thermogenesis), fatty acid uptake and/or oxidation, intracellular triglyceride (TG) mobilization,
mitochondrial content, or sympathetic activity. CT, computed tomography; DFA, dietary fatty acids; 18FDG, 18F-fluoro-deoxyglucose; FSF, fat signal
fraction; 18FTHA, 18F-fluoro-thia-heptadecanoic acid; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NEFA, nonesterified
fatty acids; PET, positron emission tomography; TG, triglycerides; TSPO, translocator protein.Endocrine Reviews, 2022, Vol. XX, No. XX 9
and mice, in which classic brown adipocytes and recruit- nerves participate in the control of lipolysis by sensing lipo-
able beige fat cells typically develop in iBAT and inguinal lytic products (223). Local neuronal sensing of WAT lipolysis
WAT (ingWAT), respectively (207, 208). In both iBAT and and/or change in temperature may contribute to activating
ingWAT, Ucp1-expressing adipocytes are under major sym- BAT sympathetic activity through afferent nerve signaling to
pathetic nervous system (SNS) control (201, 209, 210) and, the brain (223, 224). Of note, iBAT appears under a weaker
accordingly, these cells are surrounded by a very high density sensory control than WAT (218, 219).
of SNS nerve-ending varicosities, which, in contrast, are only The brain centers and circuits governing the SNS outflow
sparsely present in Ucp1-deprived white adipocytes (211, to BAT and WAT are largely determined by 2 major processes,
212). An anabolic role for parasympathetic efferent vagal namely, body temperature and body energy homeostasis,
signal, with insulin-mediated increased in glucose and fatty whose brain regulations are fairly distinct.
Downloaded from https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnac015/6594707 by guest on 19 October 2022
acid uptake and stimulation of leptin synthesis, has been sug- Body temperature regulation entangles an extensive LPB-
gested in WAT in rodents (213). However, others failed to find POA-DMH/DHyA-RPa-IML-SNS outflow pathway re-
immunohistochemical evidence for parasympathetic innerv- sponsive to cold and heat stimuli that controls the activity
ation of adipose tissues (214). Furthermore, iBAT and most of both iBAT and ingWAT to ensure body temperature sta-
other BAT depots in rodents do not display histological evi- bility (thermoregulatory thermogenesis) (202). The activation
dence of cholinergic postganglionic parasympathetic nerves of this pathway by cold exposure increases the thermogenic
(215). Therefore, the consensus is that the parasympathetic capacity in both fat depots. Of note, the thermogenic activity
nervous system plays no substantial role in adipose tissues of ingWAT, at least in mice (147), appears limited, even in
(210). As for cortical involvement in thermoregulation, iBAT-denervated cold-exposed animals in which iBAT be-
studies performed in rodents suggest it is limited to behav- comes functionally inoperative and in which ingWAT cap-
ioral thermoregulatory responses to the perception and dis- acity is enhanced (225). The current models propose that the
crimination of cutaneous temperature, without triggering POA, through excitatory glutamatergic neurons and inhibi-
of the thermoeffector responses, including BAT metabolism tory GABAergic, controls the activity of sympathoexcitatory
(198). neurons found in the DMH that project to the RPa to ul-
The SNS efferent pathways, which innervate iBAT and timately influence the activity of the brown adipocytes (226).
ingWAT distinctly, have recently further been delineated in Concretely, skin cooling leads to the stimulation of excita-
studies carried out in mice (211, 212, 216). Those studies tory LPB glutamatergic neurons, which project to the POA,
have elegantly demonstrated that murine iBAT is innerv- to trigger the concurrent stimulation of median preoptic nu-
ated by SNS preganglionic neurons emerging from the cleus excitatory glutamatergic neurons and inhibition of the
intermediolateral column (IML) of the spinal cord at the medial preoptic area inhibitory GABAergic neurons (226).
levels of the thoracic vertebrae T2 to T8, which synapse with This results in stimulation of the DMH sympathoexcitatory
postganglionic neurons found in the stellate and sympathetic neurons, which in turn excite the RPa neurons innervating
chain ganglia T2 to T5 (212, 216). For its part, ingWAT is SNS-mediated brown and beige fat depots (226).
innervated in mice by SNS preganglionic neurons leaving the BAT and WAT thermogenesis are involved not only in
IML from T7 to the first lumbar vertebra (L1), which con- temperature regulation but also likely participate in energy
nect with postganglionic neurons emerging from the thoracic/ homeostasis, which is acknowledged at least in small mam-
lumbar chain ganglia T12, T13, and L1 (211). mals. Food restriction (energy shortage) and overfeeding (en-
The brain autonomic centers governing the SNS-mediated ergy surfeit) have been reported to respectively reduce and
activity of brown/beige adipocytes are essentially located stimulate thermogenesis, thereby altering energy expenditure
in the hypothalamus and brainstem (200–204), which are to achieve the stability of energy stores (1). The observation
known to participate in most homeostatic regulations. The that iBAT (227) and ingWAT (228) polysynaptically con-
hypothalamic structures involved in the brain control of SNS- nect to hypothalamic and brainstem centers implicated in the
mediated function of UCP1-expressing adipocytes include regulation of energy balance and the direct demonstrations
the preoptic area (POA), dorsomedial hypothalamus (DMH), that those centers govern the activity of iBAT and ingWAT
dorsal hypothalamic area (DHyA), arcuate nucleus (ARC), tend to further support a genuine role for brown and beige
paraventricular hypothalamus (PVH), lateral hypothalamus adipocytes in energy homeostasis regulation. The main brain
(LH), and ventromedial hypothalamus (VMH). Neurons structures involved in such regulation include the ARC, PVH,
from those structures connect to the SNS outflow via the DMH, and VMH (200, 204, 229). Those nuclei accommodate
spinal cord IML column either directly or via premotor brain- neurons that control energy intake and energy expenditure,
stem nuclei such as the raphe nuclei, which include the raphe while responding to homeostatic signals informing about en-
pallidus (RPa) (200–204). ergy balance status, which include variations in the leptin,
Most areas implicated in the SNS control of iBAT and insulin, and ghrelin levels (230–232). Those neurons are ar-
ingWAT, namely the POA, RPa, PVH, DMH, LH, and brain- ranged in circuits or systems assembled to regulate energy re-
stem nuclei, receive sensory inputs from the fat depots, serves (233).
pointing toward an SNS outflow-sensory feedback loop that One key energy homeostasis regulator, which appears to
exists both in iBAT and ingWAT depots to regulate thermo- be particularly important in the control of iBAT and ingWAT
genesis and NEFA mobilization, respectively (217–221). The activities, is the brain melanocortin system (BMS) (234–237).
sensory innervation of iBAT, whose removal induces the This system essentially consists of distinct neuropeptidergic
atrophy of the tissue (222), significantly contributes to the ARC neurons that either express proopiomelanocortin
control of thermogenesis by signaling the brain about iBAT’s (POMC) or agouti-related peptide (AgRP) and neuropep-
thermal status (222), blood flow (219), or intracellular lipo- tide Y (NPY) as well as widely distributed brain neurons that
lytic activity (209). In WAT (including ingWAT), sensory express the melanocortin receptors 3 (MC3R) and MC4R.You can also read