Pathological Investigations of Experimental Leptospirosis in Hamsters
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
Pathological Investigations of Experimental Leptospirosis in Hamsters
Zafer ÖZYILDIZ1*, Yılmaz AYDIN2
1
Harran University, Faculty of Veterinary Medicine, Department of Pathology, Sanliurfa, Turkey
2
Ankara University, Faculty of Veterinary Medicine, Department of Pathology, Ankara, Turkey
Geliş Tarihi: 22.02.2013 Kabul Tarihi: 15.04.2013
Abstract: Forty-eight Syrian hamsters (Cricetus auratus), aged 1.5-2 months, were used in this study. Forty of the animals
were infected with Leptospira interrogans serovar grippotyphosa by intraperitoneal route. Eight hamsters were given
physiological saline solution by same route. A systematic necropsy was performed on 9 animals that died during the study
period and on 31 animals that were euthanized on days 7, 14, 21 and 28 of the trial. Remaining 8 animals was necropsied
on day 28 as control group. Macroscopically, the hamsters included in Group 1. presented with mild icterus and marked
anaemia throughout the study period. The lungs were swollen and dark red coloured, and some of the cases displayed
haemorrhagic lesions. In Group 2., the liver was swollen and dark red-coloured and the kidneys were swollen and pale.
Groups 3. and 4. presented with multiple greyish white foci that varied in number, in the kidneys. Microscopically,
Hematoxylin-Eosine (HE) staining in Group 1. demonstrated the presence of haemorrhages in multiple organs, including
primarily the lungs, as well as degenerative alterations, necrosis and mild neutrophil leukocyte and mononuclear cell
infiltrations in the liver, and degenerative changes in the kidneys. In Group 2., degenerative changes and mononuclear cell
infiltrations in the liver were observed to be of greater intensity. In Groups 3. and 4., lesions were generally limited to the
kidneys and findings related to interstitial nephritis were observed. The presence of the infectious agent was detected in all
trial groups, by the silvering methods and Avidin-Biotin Complex Peroxidise (ABC-P) staining.
Keywords: Experimental, hamster, Leptospira grippotyphosa, pathology
Hamsterlerde Deneysel Leptospirosiste Patolojik İncelemeler
Özet: Çalışmada; 1,5-2 aylık, 48 adet Suriye hamsteri (Cricetus auratus) kullanıldı. Hayvanlardan 40’ına intraperitoneal yolla
L. interrogans serovar grippotyphosa verildi. Ayrıca 8 hamstere sadece steril fizyolojik tuzlu su verilerek kontrol amaçlı
kullanıldı. Deney süresince ölen 9 hayvan ve 7., 14., 21. ve 28. günlerde ötenazi uygulanan 31 hayvanın sistemik nekropsileri
yapıldı. Kalan 8 hayvan ise nekropsileri yapıldıktan sonra kontrol grubu olarak kullanıldı. Makroskobik olarak 1. gruptaki
hayvanlarda deney süresince hafif sarılık ve belirgin anemi gözlendi. Akciğerler şişkin ve koyu kırmızı renkli olup, bazı
olgularda kanamalı lezyonlara rastlandı. İkinci grupta; karaciğerler şişkin ve koyu kırmızı renkli, böbrekler şişkin ve solgundu.
Üçüncü ve dördüncü gruplarda; böbreklerde değişen sayıda küçük boz-beyaz odaklara rastlandı. Kontrol grubu hayvanlarda
ise herhangi bir makroskobik bulgu gözlenmedi. Mikroskobik olarak; grup 1’de HE boyamalarda, akciğerler başta olmak
üzere birçok organda kanamaların yanı sıra, karaciğerde dejeneratif değişiklikler ve nekrozlar ile hafif nötrofil lökosit ve
mononüklear hücre infiltrasyonlarına, böbreklerde ise dejeneratif değişikliklere rastlandı. Grup 2’de ise karaciğerde
dejeneratif değişiklikler ve mononüklear hücre infiltrasyonlarının yoğunluğunun arttığı gözlendi. Grup 3 ve 4’te lezyonların
çoğunlukla böbreklerle sınırlı kaldığı ve intersitisyel nefritise ilişkin bulguların şekillendiği dikkati çekti. Etkenler tüm deneme
gruplarında gümüşleme metotları ve Avidin-Biotin Kompleks peroksidaz boyamalarla dokularda tespit edildi.
Anahtar Kelimeler: Deneysel, hamster, Leptospira grippotyphosa, patoloji
Introduction Many studies have been conducted on the
pathological findings, diagnosis and treatment of
Leptospirosis is a spirochetal and zoonotic the disease in hamsters by the use of several
disease of domestic and wild animals caused by serovars (Barnett et al., 1999; Haake, 2000;
Leptospira interrogans serovars (Ellis et al., 1994; Matsuo et al., 2000; Sitprija et al., 1980; Van Den
Arda et al., 1997; Hazıroğlu and Milli, 2001; Jones Ingh and Hartmann, 1986; Weber et al., 1956).
et al., 1997). Leptospirosis is a major cause of There have been more studies about light and
mortality from acute disease, characterized by electron microscopic investigation of pulmonary
septicaemia, hepatitis, nephritis and meningitis, as haemorrhages caused by the serovar
well as of aborts and stillbirth, in farm animals grippotyphosa (Berkin, 1982) and the investigation
(Badiola et al., 1983; Ellis et al., 1983; Hazıroglu of liver and kidney lesions caused by experimental
and Milli, 2001; Krivoshein, 1989). infection with several serovars, including L.
Leptospirosis is one of the most common grippotyphosa (Haake et al., 2000; Miller and
diseases in the world, which is considered Wilson, 1966; Scanziani et al., 1989; Schricker and
important in terms of both human and animal Hanson, 1961;). Recently there have been many
health and economic losses (Jones et al., 1997). studies about immunohistochemical investigation
18 Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
of leptospirosis (Haake, 1999; Haake et al., 2000; performed such that the final concentration was 1-
8
Yener and Keles, 2001). 2 x 10 /ml. Forty of the hamsters were injected
The present study was aimed at the detailed with 0.5 ml of the medium containing the L.
investigation of the clinical and pathological interrogans serovar grippotyphosa strain at a
8
findings and pathogenesis of leptospirosis in a concentration of 1x10 , while 8 of the animals
hamster experimental infection model established were administered with an equal volume of sterile
by administering the animals with L. interrogans physiological saline by intraperitoneal route as
serovar grippotyphosa by intraperitoneal route. following the decision of the Ethics Committee
(Ankara University Faculty of Veterinary Medicine
Commission Headship of the Ethics Committee
Materials and Methods 24.07.2002 - 2002/39).
The anti-Leptospira interrogans serovar
Animals, infectious agent and hyperimmune sera: grippotyphossa serum, used as the hyperimmune
The infectious agent, experimental animals and serum for immunoperoxidase stainings, was
hyperimmune serum used in the study were obtained by the administration of Leptospira
obtained from the Etlik Central Veterinary Control interrogans serovar grippotyphossa antigens
and Research Institute. Forty-eight Syrian hamsters (Strain RM52) to rabbits in the laboratories of the
(Cricetus auratus), aged 1.5-2 months and same institute. The experimentally infected
weighing 120-150 g, were used. animals were allocated to four groups, which were
To increase the number and pathogenicity of examined on days 7, 14, 21 and 28 of the trial,
the infectious agent, the serovar was passaged in respectively. Immediate post-mortem examination
the laboratories of the Institute using modified was performed on animals that died within the
Johnson’s synthetic medium (25 °C, pH 7.5) trial period, and animals that survived were
[Leptospira medium base EMJH (Bacto)] and was euthanized for necropsy. Necropsy findings were
incubated at 30 °C over a period of 4-14 days. The recorded. The numbers of animals that died within
viable Leptospira bacteria obtained by passaging the study period and that were euthanized for
were counted using a Thoma haemocytometer necropsy are presented in Table 1.
slide (Hawksley, London), and dilution was
Table 1. Numbers of animals that died and were euthanized for necropsy.
Groups Mortality Euthanasia Control
Trial Control
Group 1. ( Days 1-7) 8 2 2 10 2
Group 2. (Days 8-14) - 10 2 10 2
Group 3. (Days 15-21) 1 9 3 10 2
Group 4. (Days 22-28) - 10 3 10 2
Total 40 8
Pathology: Macroscopic findings observed in the serum was used for the detection of leptospiral
hamsters on which systematic necropsy was antigens in the sampled tissues. The Avidin-Biotin
performed were recorded. Tissue samples taken Complex Peroxidase (ABC-P) method was applied by
from the organs were fixed in 10% buffered the use of an anti-rabbit universal kit (DAKO,
formalin solution. Following fixation, tissue samples Cytomation LSAB2, System HRP, Code: K0672). For
were subjected to routine processing and this purpose, as was for histopathological
embedded in paraffin. Sections of 5-7 μm thickness examination, sections were prepared from the
were cut from the paraffin blocks and were stained paraffin tissue blocks pertaining to each animal.
with Hematoxyline and Eosine H&E (Presnell and Subsequently, the anti-Leptospira interrogans
Schreibman, 1997). serovar grippotyphosa hyperimmune serum, which
Furthermore, to ensure improved observation was obtained from rabbits and used as the
of the infectious agent, all tissues were also stained hyperimmune serum (microscopic agglutination
with the Warthin-Starry and Levaditi methods test, 1/1200), was diluted at a rate of 1/64 and
(Presnell and Schreibman, 1997). applied to the sections. At the final stage, the
Immunohistochemistry: An anti-Leptospira sections were kept in an AEC chromogen solution
interrogans serovar grippotyphosa hyperimmune (DAKO Corporation, USA) for 7 minutes and
Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013 19Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
counterstained with Mayer’s haematoxylin for 1 foci that did not exceed a few millimetres in
minute, and rinsed under running tap water. Finally, diameter and were scattered across the hepatic
the sections were applied a water-based adhesive lobes (Figure 1b). It was noted that the kidneys
and covered with a coverslip. Tissue sections were swollen and friable and had a mottled
belonging to the control group used as negative appearance (Figure 1c). Sand-like greyish white foci
controls were subjected to the same procedure. were scattered across the cortex. The prescapular
and popliteal lymph nodes were enlarged and their
Results cross sections had a moist appearance. Animals
included in Group 4. presented with swelling and
paleness of the liver and a mottled appearance of
Clinical Findings the kidneys. In some of these animals,
As from day 2 of the trial, the animals indistinguishable greyish white foci, some of which
presented with various clinical symptoms, including reached the size of sand particles, were present
inertia, ruffled it’s feathers, spinal curvature below the capsule (Figure 1d). In the kidneys, the
(hollow-back), listlessness, inappetence, slowness of proportion of the cortex to the medulla was
movements, delayed reaction to physical stimuli, observed to have increased in favour of the cortex.
reduced interest in feed and water consumption, No macroscopic finding was observed in the control
huddling near the sides of the cages and the animals that were necropsied in pairs on days 7, 14,
tendency to acquire dorsal recumbency. At the end 21 and 28 of the trial.
of day 2, two of the animals were observed to have
died. Some of the animals that died on days 4 and 5 Microscopic Findings
displayed tonic-clonic convulsions in unconscious As the microscopic findings varied with the
state and dorsal recumbency shortly before death. different phases of the infection and the severity of
As from days 6 and 7, the animals were observed to the lesions, the organs with the most severe lesions
show increased interest in water and feed were examined in the first place. In this respect, the
consumption. The clinical symptoms were less infection was investigated under three phases,
severe in Group 2., whilst Groups 3. and 4. did not namely, the acute destructive, subacute and chronic
show any clinical symptom except from one dying in phases.
Group 3. 1. Acute destructive phase: In this phase, the
organs that were affected at the highest level by
Necropsy Findings the infectious agent after septicaemia, and the
Most pronounced necropsy finding was damage associated with infection, were examined.
petechial haemorrhage on the serous membranes Group 1. was examined in this phase of the
of the internal organs in the 8 animals that died infection. The animals in Group 1. demonstrated
naturally and the 2 animals that were sacrificed end the septicaemic phase of the infection and
of the 7 th days of the trial. In all of the cases, the presented with evident and characteristic
lungs were dark red in colour and swollen. In 3 of pulmonary lesions; including, hyperaemia of the
the animals, the cranial and caudal lobes have interalveolar capillaries and interlobular arteries
displayed multifocal petechial and ecchymotic and veins, diffuse haemorrhagic areas and mild
haemorrhage (Figure 1a). The 3 animals that died acute catarrhal bronchopneumonia. The Levaditi,
on day 5 presented with a aspect of mild icterus in Warthin-Starry method and immunoperoxidase
body fat, the peritoneum and subcutaneous tissues. stainings demonstrated the presence of the
The blood was light red coloured and showed weak infectious agent and it’s antigenic structure in the
clotting activity. In this trial group, the liver was lumen and wall of interalveolar capillaries,
enlarged, dark red-coloured, swollen and rounded haemorrhagic areas and the alveolar wall. In the
edge, and it was observed that blood leaked from heart, which presented with no macroscopic
the cross sections. The kidneys were also swollen finding, hyperaemia of the blood vessels was
and dark red-coloured with marked congestion of observed, and in almost each case the muscle
the subcapsular vessels, and oedema and bundles were swollen and their cytoplasm displayed
congestion of the cross sections. In Groups 2. and 3. a granular appearance. In three of the animals, in
the liver and kidneys were swollen and pale. In some areas free erythrocytes were observed
Group 3., one animal died and presented with between muscle bundles.
multiple pinhead-sized yellowish white, confined
20 Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
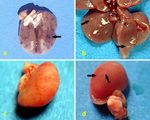
Figure 1. Macroscopical aspects: a) Lungs, Group I, Diffuse petechial and ecchymotic hemorrages (Arrow), b) Liver, Group
III, multifocal gray-white areas dispersed in lobs (Arrows), c) Kidney, Group III, Pale and multicolored appearance, d) Kidney.
Group IV, multicolored appearance and gray-white focuses in shape of grits (Arrows).
In Group 1., the vena centralis, vena proteinaceous material and epithelial debris. In
interlobularis and sinusoids were observed to have three cases, aggregates of free erythrocytes were
been greatly enlarged, and in some areas observed in the interstitium. The Levaditi method
thrombosis had occurred. The Remark cords were and Warthin-Starry stainings demonstrated at
dissociated, the sinus endothelium was swollen, pulmonary haemorrhages and the presence of
and the Kupffer cells were hyperplastic. In some of brown-black coloured aggregates of the infectious
the animals, apart from the listed findings, granular agent that resembled a spiral in shape, in the
degeneration and solitary cell necroses were bronchiolar epithelium, interalveolar capillaries,
observed in hepatic epithelial cells. Surrounding hepatic sinusoids, Disse spaces, renal glomeruli and
these lesions, cell infiltrations, composed generally proximal tubuli. Furthermore, many
of macrophages and lymphocytes, and to a less immunopositive areas were detected by
extent, neutrophils, were observed. Similar immunoperoxidase staining in the glomeruli,
inflammatory cells were also present in small lumen of the intertubular arteries and proximal
numbers in the sinusoids. Immunoperoxidase tubules, as well as in the hepatic sinusoids, Disse
staining demonstrated the presence of the spaces and cytoplasm of the Kupffer cells. No
antigenic structure in this regions. immunopositivity was detected in any of these
Renal findings were more pronounced in regions in the control group.
Group 1. In almost each case, marked hyperaemia Brain lesions were not specific, but in one
of the renal blood vessels and evident glomerular animal, aggregates of free erythrocytes were
alterations were observed. Enlargement of the observed in the submeningeal tissue, which
glomerular capillaries and Bowman’s space, extended towards the neuropile.
proliferation of the mesangial cells, the presence of Findings observed in the spleen included
proteinaceous materials in Bowman’s space hyperplasia of the lymph follicles, oedema in the
associated with free erythrocytes in some regions trabeculae, infiltrations mainly composed of
were also determined. The proximal convoluted macrophages and also a few neutrophil leukocytes,
tubule epithelium contained fine granules and light and marked increase in the red pulp.
coloured vacuolisation in certain regions. In some In the prescapular, popliteal and mesenteric
of the tubules, the lumen contained aggregates of lymph nodes, the cortical and medullar sinuses
Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013 21Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
were observed to have enlarged and their lumen The control stainings did not produce any
contained macrophages and a few neutrophil immunopositive area.
leukocytes. In some animals free erythrocytes were 3. Chronic regenerative-proliferative phase:
present in the parenchyma. Groups 3. and 4. were examined in this phase of
2. Subacute phase: This phase was characterized the infection. The hepatic lesions observed in the
by the continuation of destructive processes at a animals included in Group 3. differed from those
progressively decreasing level and the observed in Groups 1. and 2. Findings related to
strengthening of the immune response. Group 2. hepatic degeneration continued but with
was examined in this phase of the infection. progressive decrease, while the dissociation of the
In the animals included in Group 2., the Remark cords and regenerative alterations in the
organs most affected by the infection were the hepatic epithelial cells had increased. It was
liver and kidneys. Degeneration and necrosis were determined that the number and concentration of
again observed in the liver, and mononuclear cell the mononuclear cell foci found in the periportal
foci of greater number and concentration were and periacinar regions had decreased. In some
present in the parenchyma. In almost each case, areas the bile ducts were hyperplasic. The number
apart from vacuolar degeneration, mononuclear of hepatocytes with double and large nuclei had
cells were observed, which were mainly composed increased.
of macrophages and also contained a few Renal findings also differed from those
neutrophil leukocytes, and were ascertained to observed in the first two groups. With respect to
have formed 2 or 3 foci in a single microscopic field the distribution of lesions, glomerular alterations
(X10 objective). The foci contained necrotic were of less importance and existing lesions were
hepatocytes in the centre and were located mostly at tubular-interstitial level. Intracytoplasmic
particularly in the periportal and periacinar regions vacuoles and desquamation were still observed in
(Figure 2a). The vena centralis, portal veins and the lumen of the tubules, but interestingly, cell
sinus endothelium were swollen and contained infiltrations had started to emerge in the
macrophages in their lumen. The interstitium. Cell infiltrations were observed
immunoperoxidase technique produced generally around the subcapsular, periglomerular
immunopositive stainings (Figure 2b). Control and convoluted tubules, as well as around the
stainings produced no immunopositive area. arteries in the corticomedullar region, and were
Renal lesions were characterized mostly by observed to be less severe than those observed in
glomerular alterations, proximal tubular Group 4. Immunoperoxidase stainings produced
degeneration and necrosis. It was important point immunopositive areas in the sinusoids and the
that the tubular degenerative alterations had sinusoid-facing surface of epithelial cells in the
developed particularly in a few tubules liver; and in the tubular epithelial cells of the
surrounding the glomeruli. In these areas, tubular cortex and cytoplasm of the macrophages in the
epithelial cells were swollen and their cytoplasm interstitium in the kidneys. Control stainings did
was light coloured. Some of these cells contained not produce any immunopositive area.
vacuoles with indefinite periphery and displayed In Group 4., nonsuppurative interstitial
patches of pink coloured granules (Figure 2c). In nephritis, a typical symptom associated with the
some areas, the lumen of the tubules contained disease, was observed at varying intensity in all
epithelial debris and proteinaceous materials. animals, and was much severe than that observed
The Levaditi method and Warthin Starry in Group 3. The liver displayed both degenerative
staining demonstrated the presence of the and regenerative findings, and periportal and
infectious agent in the form of masses in hepatic periacinar mononuclear cell infiltrations were
sinusoids and Disse spaces, and also as adhered to either very scarce or did not exist.
the tubular epithelium in the proximal and distal Although renal lesions did not display
tubules of the kidneys. Immunoperoxidase significant in-group differences, the distribution
stainings produced immunopositive areas on the and severity of the lesions varied. The lesions were
sinusoidal surface of hepatocytes, within sinusoids, predominated by interstitial nephritis, which varied
and in the cytoplasm of Kupffer cells and from subacute to chronic in course. In the
macrophages in the liver; and in the proximal periphery of the proximal and distal convoluted
tubules (Figure 2d), the lumen of intertubular tubules and interlobular arteries found in the
blood vessels and areas visualized with pycnotic periglomerular region, both interstitial oedema
nuclei in the corticomedullar region in the kidneys. and mononuclear cell infiltrations were observed.
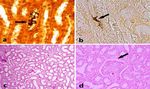
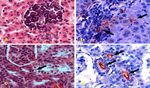
22 Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article Figure 2. Histopathological wiew of sections for H&E and Immunohistochemical staining. a) Liver, Group II, focal mononuclear cell infiltrations in parenchyma, H&E, X400, b) Liver, Group II, immunopositive stainings in cytoplasms of mononuclear cells and sinusoids, (Arrows), ABC-P, X 400, c) Kidney, Group II, vacuoler and hydrophic degenarations in proximal tubules in areas near glomerulus (Arrow), H&E, X400, d) Kidney, Group II, immunopositive stainings in tubule lumens around glomerulus (Arrows), ABC-P, X 400. Figure 3. Histopathological wiew of sections for H&E, Levaditi and Whartin Starry staining. a) Kidney, Group IV, spiral shaped leptospiras in piles hanging on tubule lumens (Arrow), Levaditi X400, b) Liver, Group IV, agents hanging tubule epithels (Arrow), Warthin- Starry, X1000, c) Kidney, Group IV, connective tissue proliferations in interstitium and proteinaceous deposits in tubule lumens, H&E, X100, d) Kidney, Group IV, thickening in parietal leaf of Bowman capsule (Arrow), H&E, X400. Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013 23
Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
The Levaditi method and Warthin-Starry haemorrhages associated with leptospirosis are
stainings demonstrated the presence of the spiral- caused by ischemia.
shaped infectious agent, mostly in the form of Some researchers have reported that the
clusters, in the epithelial cells and lumen of the haemorrhages observed particularly in the lungs
tubules as well as in the interstitium (Figure 3a, and the serous membranes (Miller et al., 1974),
3b).In some of the euthanized animals, thickening and the renal lesions (Van Den Ingh and Hartman,
and partial adhesion of the glomerular basal 1986) associated with leptospirosis, develop as a
membrane were observed (Figure 3d), whilst some result of the damage to blood vessel walls and
of the glomeruli were atrophic. Furthermore, mild thromobosis caused by leptospiral toxins (Arean et
proliferation of the connective tissue in the al., 1964). However, in the present study, Warthin-
interstitium and the presence of pink homogenous Starry and immunoperoxidase stainings having
proteinaceous materials in part of the cortical demonstrated haemorrhagic areas in the lungs and
tubular epithelium were observed (Figure 3c). In the presence of the infectious agent and it’s
two of the animals, syncytial cell formations, which antigenic structure in the interalveolar capillaries
were composed of sloughed epithelial cell groups, and alveolar epithelium, the renal glomerular and
were observed in the cell infiltrations located in tubule epithelium in the kidneys, as well as in other
the interstitium. organs in the animals included in Group 1.;
Immunoperoxidase stainings produced a few together with the observation of interstitial cell
weakly stained areas in the liver of some of the infiltrations and immunopositive areas in these
animals. No immunopositive stainings were cells in Groups 3. and 4., supported the opinion
determined in the other animals. In the kidneys, that lesions are caused by the infectious agent
immunopositive areas were determined within itself (Barnett et al., 1999; Pereira et al., 1997;
macrophages and in the lumen of tubules. The Sitprija et al., 1980).
control stainings did not yield any immunopositive Another aspect that has attracted the
area in the liver or kidneys. attention of researchers in the past years is the
route the leptospiral agents use to reach the lumen
Discussion of renal tubules. While some researchers have
suggested that the infectious agent passes from
As a spriochetal infection that threatens both the blood vessels to the interstitium and from the
human and animal health, leptospirosis has been interstitium to the lumen of the tubules (Alves et
known for more than a century. Various researches al., 1991; Sitprija et al., 1980), some other have
have been conducted on the pathogenesis, claimed that the infectious agent passes into the
diagnosis and treatment of the disease (Abdu and interstitium from the lumen of the tubules and
Sleight 1965; Hubbert and Miller, 1967; Barnett et triggers the interstitial reaction (Barnet et al.,
al., 1999; Haake et al., 2000) and multiple methods 1999). Researchers have not been able to fully
have been developed for protection against the explain how the causative agent passes the
infection (Avila et al., 1985; Brenot et al., 2001; epithelial barrier, but have put forward several
Matsuo et al., 2000). The pathogenesis of the theories. Barnet et al., (1999) have suggested 3
lesions caused by leptospiral agents has drawn the theories. According to the first, by causing the
attention of researchers for many years and the degeneration of the tubular epithelium, the
focus of previous research has been the infectious agent increases the permeability of the
investigation of whether the lesions associated basal membrane, and thereby, passes into the
with the disease are caused by the infectious agent interstitium. On the other hand, the second theory
itself. As regards this issue, some researchers have suggests that the agent has access to the
claimed that the lesions result from the damage cytoplasm of epithelial cells by means of its spiral
caused by the spiral movements of the leptospiral movements, and from here is passed into the
agents to the blood vessel wall and tissues (Barnett interstitium via intracytoplasmic vacuoles by
et al., 1999; Haake et al., 2000; Pereira et al., 1997; means of intracellular transport. According to the
Sitprija et al., 1980;), while some other researchers third theory, the agent passes from the tubular
(Miller et al., 1974; Van Den Ingh and Hartmann, epithelial cells into the interstitium by active
1986) have suggested that immunoglobulins transport.
produced against leptospiral exotoxins activate the In the present study, immunoperoxidase
complement system, and thereby lead to the stainings in Group 2. having produced
degranulation of neutrophil leukocytes, which immunopositive areas that appeared as granules in
brings about the breakdown of the wall of blood the cytoplasm of renal tubular epithelial cells, and
vessels and the formation of thrombosis, and have the inexistence of positive staining in the vacuoles
thus, claimed that the degeneration and found within these epithelial cells, do not conform
24 Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
with the claim of Barnet et al., (1999) that “the the blood vessels in the interstitium towards the
infectious agent passes into the interstitium within tubules, and have also indicated the inflammatory
intracytoplasmic vacuoles”; but rather suggest that reaction to result from the movement of the
these vacuoles could be signs of acute cellular infectious agent in the interstitium. It was
swelling resulting from the effect of leptospiral noteworthy that in Groups 1. and 2., the first
agents on the tubular epithelium. Furthermore, as region that was affected by the disease was the
leptospiral agents have the capability of rotational tubular epithelium in the periphery of the
movement (Arda et al., 1997) and given that their glomeruli. In this phase, mononuclear cell
residues are toxic (Miller et al., 1974; Sitprija et al., infiltrations were not encountered in the
1980; Masuzawa et al., 1990), the theory that interstitium. Leptospiral antigens were detected in
these agents pass from the tubular epithelial cells the glomeruli, the walls surrounding the lumen of
into the interstitium by active transport and adjacent tubules, and the wall, lumen and
without causing any damage to cells is not periphery of interstitial blood vessels. These
considered likely. findings suggest that the infectious agent may
Researchers, who have detected the presence access the interstitium by both blood vessels and
of leptospiral antigens in the periphery of renal tubules, and that apart from the agent and its
interstitial blood vessels within the first 3 hours of residues, the degeneration of the tubules and
infection, the lumen of tubules by the end of the blood vessels may also have influence on the
th
9 hour of infection (Sitprija et al., 1980), in free development of the interstitial reaction.
form in the lumen of blood vessels and the The findings other than haemorrhage, which
interstitium in the corticomedullar region on the were observed in the other organs, were not
th
4 day of infection, within macrophages in the considered as typical symptoms of the disease, and
interstitium, in the periphery of tubules, in the were assessed as nonspecific findings.
tubular basal membranes and tubular epithelial
th
cells on the 5 day of infection, and in the lumen Acknowledgement: This manuscript is summerised
th
of tubules on the 6 day of infection (Alves et al., from doctora thesis (Hamsterlerde Deneysel
1991) have reported the distribution of the Leptospiroziste Patolojik İncelemeler) in Ankara
infectious agent in the renal tissue to occur from University Institue of Health Science, 2006.
References
Abdu MTF, Sleight SD, 1965: Pathology of experimental
Barnett JK, Barnett D, Bolin CA, Summers TA, Wagar EA,
leptospira pomona infection in hamsters. Cornell
Cheville NF, Hartskeerl RA, 1999: Expression and
Vet, 55, 74–86.
distribution of leptospiral outher membrane
Alves VA, Gayotto LC, Yasuda PH, Wakamatsu A,
components during renal infection of hamsters. Infect
Kanamura CT, De Brito T, 1991: Leptospiral
Immun, 67, 853-861.
antigens (L. interrogansserogrup ictero-
Berkin Ş, 1982: Hamsterlerde deneysel leptospiroziste
haemorrhagiae) in the kidney of experimentally
(L.grippotyphosa) akciğer kanamalarının ışık ve
infected guinea pigs and their relation to te
elektron mikroskobik incelenmesi. Ankara Üniv Vet
pathogenesis of the renal injury. Exp Pathol, 42,
Fak Derg, 29,188-205.
81–93.
Brenot A, Trott D, Girons IS, Zuerner R, 2001: Penicillin-
Arda M, Aydın N, Ilgaz A, Minbay A, Kahraman M, İzgür
binding proteins in Leptospira interrogans. Antimicrob
M, Leloğlu N, Akay Ö, Diker KS, 1997: Spiroketler.
Agents Chemoter, 3, 870-877.
In: Veterinary Microbiology. Ed.: Mustafa Arda,
Ellis WA, Bryson DG, Neill SD, Mcparland PJ, Malone FE,
Medisan Press, Ankara, Turkey. pp. 257-274.
1983: Possible involvement of leptospires in abortion,
Arean VM, Sarasin G, Green JH, 1964: The pathogenesis
stilbirths and neonatal deaths in sheep. Vet Rec, 112,
of leptospirosis: toxin production by Leptospira
291-293.
icterohaemorrhagiae. Am J Vet Res, 25, 836-842.
Ellis GR, Partington DL, Hindmarsh M, Barton MD, 1994:
Avila FA, Bechara GH, Santa Rosa CA, Avila SHP, Santana
Seroprevalence to Leptospira interrogansserovar
AE, 1985: Evaluation of the immune humoral
hardjoin merino stud rams in south Australia. Aust Vet
response in swine experimentally vaccinated
J, 71, 203- 206.
against Leptospira interrogans serotypes pomona
Haake DA, 1999: Expression and distribution of leptospiral
and canicola. Ars Vet, 1, 51-55.
outher membrane components during renal infection
Badiola J, Thiermann AB, Cheville NF, 1983: Pathologic
of hamsters. Infect Immun, 67, 853-861.
features of leptospirosis in hamsters caused by
Haake DA, 2000: Spirochaetal lipoproteins and
Leptospira interrogansserovars hardjoand
pathogenesis. Microbiol, 146, 1491–1504.
szwajizak. Am J Vet Res, 44, 91-99.
Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013 25Harran Üniv Vet Fak Derg, 2(1) 18-26;2013 Research Article
Haake DA, Chaogzuerner RL, Barnett JK, Barnett B, Mazel Pereira MM, Andrade J, Lacerda MD, Batoreu NM,
M, Matsunaga J, Levett P, Bolin CA, 2000: The Marchevsky RS, Dos Santons RR, 1997: Demonstration
leptospiral major outher membrane protein LipL32 of leptospiral antigens on tissues using monoclonal
is a lipoprotein expressed during mammalian antibodies and avidin-biotin peroxidase staining.
infection. Infect Immun, 68, 2276–2285. Exp Toxic Pathol, 49, 505–511.
Hazıroglu R, Milli ÜH, 2001: Urinary system. In: Presnell JK, Schreibman MP, 1997: Staining microorganisms.
Veterinary Pathology Eds., Hazıroğlu R, Milli UH, Humason’s animal tissue techniques. Johns Hopkins
Vol. I., Tamer Press, Ankara, Turkey. Univ. Press. Ltd., USA.
Hubbert WT, Miller JN, 1967: Effect of antibiotic Scanziani E, Sironi G, Mandelli G, 1989: Immunoperoxidase
theraphy on experimental leptospirosis infection studies on leptospiral nephritis of swine. Vet Pathol,
and development of acquired resistance in guinea 26, 442–444.
pigs. Am J Vet Res, 28, 861–864. Schricker RL, Hanson LE, 1961: Effect of cortisone on
Jones TC, Hunt RD, King NW, 1997: Veterinary Pathology. leptospira pomona infection in the guinea pig. Am J
Sixth Ed. Lippincott Wilkins, USA. Vet Res, 22, 580–586.
Krivoshein YS, 1989: Spirocethes. Handbook on Sitprija V, Pipatanagul V, Mertowidjojo K, Boonpucknavig V,
microbiology laboratory diagnosis of infection Boonpucknavig S, 1980: Pathogenesis of renal disease
disease. Mir Publishers, United Kingdom. in leptospirosis: Clinical and experimental studies.
Masuzawa T, Nakamura R, Sehımızu T, Yanagıhara Y, Kidney Int, 17, 827–836.
1990: Biological activities and endotoxic activities Van Den Ingh TS, Hartman EG, 1986: Pathology of acute
of protective antigens (Pags) of leptospira leptospira interrogans serotype icterohaemorrhagiae
interrogans. Zbl Bakt, 274, 109-117. infection in the syrian hamster. Vet Microbiol, 12,
Matsuo K, Isogai E, Araki Y, 2000: Control of 367–376.
immunologically crossreactive leptospiral infection Weber WJ, Creamer HR, Bohl EH, 1956: Chemotheraphy in
by administration of lipopolysaccharides from a hamsters cronically infected with Leptospira canicola.
nonpathogenic strain of leptospira biflexa. J Am Vet Med Assoc, 15, 271-273.
Microbiol Immunol, 44, 887–890. Yener Z, Keles H, 2001: Immunoperoxidase and
Miller KG, Wilson RB, 1966: Electron microscopy of the Histopathological Examinations of Leptospiral
liver of the hamster during acute and chronic Nephritis in Cattle. J Vet Med A, 48, 441-447.
leptospirosis. Am J Vet Res, 27, 1071–1081.
Miller NG, Allen JE, Wilson RB, 1974: The pathogenesis of *Corresponding Address:
hemorrhage in the lung of the hamster during Zafer ÖZYILDIZ
acute leptospirosis. Med Microbiol Immunol, 160, Harran Üniversitesi, Veteriner Fakültesi,
269–278.
Patoloji Anabilim Dalı, Eyyübiye Yerleşkesi,
63200, Şanlıurfa
E-mail: zaferozyildiz@hotmail.com
26 Harran Üniversitesi Veteriner Fakültesi Dergisi Cilt 2, Sayı 1, 2013You can also read