Redox Switching of Polyoxometalate Methylene Blue-Based Layer-by-Layer Films
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Article
pubs.acs.org/Langmuir
Redox Switching of Polyoxometalate−Methylene Blue-Based
Layer-by-Layer Films
Nargis Anwar,† Mikhail Vagin,† Rashda Naseer,† Shahzad Imar,† Masooma Ibrahim,‡ Sib Sankar Mal,‡
Ulrich Kortz,‡ Fathima Laffir,§ and Timothy McCormac*,†
†
Electrochemistry Research Group, Department of Applied Science, Dundalk Institute of Technology, Dublin Road, Dundalk,
County Louth, Ireland
‡
School of Engineering and Science, Jacobs University, P.O. Box 750561, 28725 Bremen, Germany
§
Materials and Surface Science Institute, University of Limerick, Limerick, Ireland
ABSTRACT: Iron-substituted crown-type polyoxometalate (POM)
[P8W48O184Fe16(OH)28(H2O)4]20− has been successfully immobi-
lized onto glassy carbon electrode surfaces by means of the layer-
by-layer (LBL) technique employing the cationic redox active dye,
methylene blue (MB). The constructed multilayers exhibit pH-
dependent redox activity for both the anionic POM and the cationic
dye moieties, which is in good agreement with their solution
behavior. The films have been characterized by alternating current
impedance, atomic force microscopy, and X-ray photoelectron
spectroscopy, whereby the nature of the outer layer within the
assemblies was found to have an effect upon the film’s behavior.
Preliminary investigations show that the POM dye-based films show electrocatalytic ability toward the reduction of hydrogen
peroxide, however, only when there is an outer anionic POM layer.
1. INTRODUCTION substrate is also another advantage of the LBL techni-
Polyoxometalates (POMs) are inorganic metal−oxygen clusters que.26,29−31
that display great diversity in both their structure and A wide range of POMs have been surface-attached through
composition.1,2 Their properties enable them to be employed the LBL technique, e.g., Wells−Dawson-type [P2W18O62]6−,
across a wide domain, including material science, medicine, Keggin-type [α-SiW12O40]4−, transition metal-substituted
catalysis, biotechnology, and nanotechnology.3−12 What is of Krebs-type POMs [X2W20M2O70(H2O)6]n−, where (X = Bi or
general interest when considering these application domains is Sb, M = Co 2+ or Cu 2+ ), and sandwich-type POMs
the ability to surface-immobilize these POMs onto a variety of [Co4(H2O)2(PW9O34)2]10−.6,32−34 A number of substrates
surfaces whereby their inherent redox and photophysical have also been employed, such as glassy carbon,6,30,32,33,35−37
properties are maintained. The various techniques utilized to highly ordered pyrolytic graphite,37 mercury, platinum, gold,36
date for surface attachment of POMs include self-assembled quartz,26,30,32,33,35,38 indium tin oxide (ITO),34,37−39 gold-
monolayers (SAMs), Langmuir−Blodgett and sol−gel films, coated quartz,37,39 silicon,38,39 and mica substrate.39 A variety
electrodeposition, entrapment into conducting polymer films, of cationic moieties have been incorporated into these POM-
and the layer-by-layer (LBL) self-assembly method.13−24 based multilayers systems, such as, ruthenium(II) polypyridyl
Electrostatic attractions and van der Waals forces are complexes, 38,40 conducting 41,42 and redox active poly-
considered to be involved during the growth of the such LBL mers,30,36,43 metallodendrimers,35 metalloporphyrins,44 poly-
layers.25 Utilizing the electrostatic attraction between oppo- electrolytes,29,32,33,39,45−47 cationic surfactants,40 dye mole-
sitely charged species,26 the LBL method is a great tool of cules,36 and various multiply charged cations.6,37,44,47
immobilization for the construction of organized multilayer Two methods are generally used to construct the multilayer
assemblies. Iler was the first to discover the method in 1966,27 assemblies onto a modified surface. The first one is immersion
and it was not until 1991 that this work was rediscovered growth, e.g., alternately dipping a solid substrate into two
through the work of Decher and Hong.28 The LBL method is solutions of oppositely charged modifiers.30,36,41−43 Electro-
both simple and efficient with functional supramolecular chemical growth involves alternate cyclic potential sweeps of
systems being easily fabricated on various surfaces by the substrate being performed in a solution of oppositely
controlling the composition, thickness, and orientation of charged species.36,44 Cyclic voltammetry,6,30,35,36,39 UV/visible
each layer at the molecular level within the assembly. These
structures show good mechanical and chemical stability, which Received: January 27, 2012
make them attractive for sensing and electronic applications. Revised: February 21, 2012
The possibility to adopt different sizes and shapes of the Published: February 22, 2012
© 2012 American Chemical Society 5480 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
(UV−vis) spectroscopy,6,26,30,32−35,37−39 and impedance spec- employment of the LBL technique. It is seen that the
troscopy30 were employed to look at the growth of multilayer characteristic redox behavior of both the POM and the MB
assemblies onto substrates. The characterization of multilayers have been maintained within the solid state. The results for the
was performed by electrochemical quartz crystal micro- electrochemical and surface properties of these nanostructured
gravimetry (EQCM), 37 scanning electron microscopy POM-based layers is discussed in detail. This contribution
(SEM),26 atomic force microscopy (AFM),32,33,35,38,39 X-ray represents the first time successful immobilization of
photoelectron spectroscopy (XPS),38,39 fluorescence spectros- [P8W48O184Fe16(OH)28(H2O)4]20−.
copy,26 Fourier transform infrared (FTIR) spectroscopy, and
electron spin resonance (ESR) techniques.39 2. EXPERIMENTAL SECTION
Different polycations and polyanions have also been adhered
2.1. Materials. The hydrated potassium-lithium salt of the crown-
to the electrode surfaces by the LBL technique using a type 48-tungsto-8-phosphate Li 4 K 16 [P 8 W 48 O 184 Fe 16 (OH) 28 -
range of ionic dyes.45−49 The combination of organic dye (H2O)4]·66H2O·2KCl (LiK- P8W48Fe16) was synthesized according
moieties, which possess high molar extinction coefficients to the literature.53 An 8% solution of poly(diallyldimethylammonium
and absorb visible light readily, with redox active POMs, chloride) (PDDA, MW 20000) was prepared from stock. All other
which are poor absorbers of visible light, can yield organic− chemicals were of reagent grade, purchased from Aldrich, and were
inorganic hybrid materials that possess unique photo- used as received unless otherwise stated. Alumina powders of sizes
catalytic properties. However, only a few examples are 0.05, 0.3, and 1.0 μm were received from CH Instruments. Water was
purified using a Milli-Q water purification system.
reported for the LBL immobilization of POMs with dye
2.2.1.Apparatus and Procedures. 2.2.1. Electrochemical
molecules.26,50 The LBL technique is employed for the Measurements. All electrochemical experiments were performed
electrode surface attachment of metal nanoparticles, which with a CHI660 electrochemical workstation employing a
can be used for detailed studies of the kinetics of electron conventional three-electrode electrochemical cell. A GCE (3
transfer to them.51 mm diameter, surface area 0.0707 cm2), a platinum wire as the
The recently reported iron-substituted POM of crown-type auxiliary electrode, and silver/silver chloride as the reference
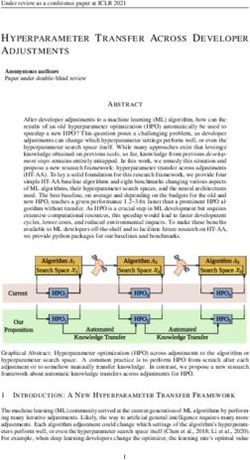
[P8W48O184Fe16(OH)28(H2O)4]20− (P8W48Fe16),52 shown in electrode (3 M KCl) in aqueous media were employed for all
Figure 1, possesses 16 equivalent iron centers, which are electrochemical measurements unless stated otherwise. The
working electrode was successively polished with 1.0, 0.3, and
0.05 μm alumina powders and sonicated in water for 10 min
after each polishing step. Finally, the electrode was washed with
ethanol and then dried with a high-purity nitrogen stream
immediately before use. Solutions were degassed for at least 20
min with high-purity nitrogen and kept under a blanket of
nitrogen during all electrochemical experiments. The following
electrolytes were used for the electrochemical experiments
involving P8W48Fe16: 0.5 M Li2SO4 (pH 2.0), 1 M LiCl (pH
1.0−3.0), and 1 M CH3COOLi (pH 3.5−7.0). The pH
adjustment was done with 0.5 M H2SO4, 1 M HCl, and 1 M
CH3COOH, respectively.
2.2.2. Construction of Multilayer Assemblies of POM and MB. A
clean GCE was immersed in the 8% (v/v) PDDA solution for 1 h for
initial surface modification (step 1). The electrode was then rinsed
thoroughly with deionized water and dipped in a 0.25 mM solution of
LiK- P8W48Fe16 in pH 2 buffer solution (0.5 M Li2SO4, 0.5 M H2SO4)
for 20 min (step 2). The electrode was then again rinsed thoroughly
with deionized water and dried with high-purity nitrogen. This process
resulted in a bilayer composed of both an underlying PDDA layer and
an outer POM layer. The bilayer (PDDA/POM) modified electrode
was then soaked for 20 min in a 0.02 mM aqueous MB solution for 20
min (step 3). The electrode was then again rinsed thoroughly with
deionized water and dried with high-purity nitrogen. This resulted in a
PDDA/POM/MB trilayer configuration on the underlying carbon
electrode. To build the desired number of layers onto the electrode
surface, steps 2 and 3 were repeated the required number of times.
Figure 1. Combined polyhedral/ball-and-stick representation of The terminal outer layer was chosen either to be an anionic POM or a
[P8W48O184Fe16(OH)28(H2O)4]20−. Color code: Fe (brown), O cationic MB layer.
(red), PO4 tetrahedra (pink), WO6 octahedra (teal). 2.2.3. Atomic Force Microscopy. LBL films were formed ITO
slides. AFM imaging was recorded with a Digital Instruments
characterized by a single multielectron wave of simultaneous Nanoscope III with tapping mode using Si 3N4 cantilever tips.
reduction in solution and with external and substitution-labile The spring constant of these pyramidal shape tips was between
12 and 103 N/m, and the size ranged between 3.6 and 5.6 mm.
coordination positions. The fast kinetics associated with the The images were analyzed using Nanotech Electronica WSxM image
electrochemical reaction and the multimetal substitutions are software.
the two main points, which make this POM an attractive 2.2.4. Electrochemical Impedance Spectroscopy (EIS). EIS was
candidate for electrocatalytic applications. carried out in 10 mM potassium ferricyanide and 10 mM potassium
ferrocyanide solution in 0.1 M KCl at a potential of +230 mV (versus
The present work focuses on the immobilization of Ag/AgCl) from 0.1 to 106 Hz employing a voltage amplitude of 5 mV.
P8W48Fe16 and the cationic redox dye methylene blue (MB) The measurement solution was freshly prepared and constantly
onto a glassy carbon electrode (GCE) surface through the degassed with nitrogen.
5481 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
2.2.5. X-ray Photoelectron Spectroscopy. LBL films were formed composed of a pH-dependent bielectronic redox process, with
on ITO slides. Analysis was performed in a Kratos AXIS 165 an E1/2 of +0.135 V with this formal potential undergoing a
spectrometer using monochromatic Al Kα radiation of energy 1486.6 cathodic shift of 60 mV/pH unit with an increase in pH, which
eV, 150 W (10 mA, 15 kV). The pass energy was 160 eV for survey
spectra and 20 eV for narrow regions. In the near-surface region, the corresponds to the involvement of two protons.50 The redox
atomic concentrations of the chemical elements were evaluated after behavior of both the P8W48Fe16 and the MB reported herein
subtraction of a Shirley-type background by considering the agrees with the literature.57,59
corresponding Scofield atomic sensitivity factors. Core level binding 3.2. LBL Films of P8W48Fe16. Cyclic voltammetry was
energies were determined using the C 1s peak at 284.8 eV as the utilized to monitor the growth of the multilayer assemblies
charge reference. The standard deviation of the peak position based upon PDDA, P8W48Fe16, and MB after the deposition of
associated with the calibration procedure was ±0.05 eV.
each molecular layer, with Figure 3 representing such growth.
3. RESULTS AND DISCUSSION Figure 3A shows the resulting cyclic voltammograms of the
multilayer assembly after the deposition of each POM anionic
The electrochemical behavior of both P8W48Fe16 and MB were layer, whereas Figure 3B shows the resulting cyclic voltammo-
studied in solution and after LBL immobilization onto GCE grams of the multilayer assembly after the deposition of each
surfaces. The growth of the POM-based films was monitored MB cationic layer. What is readily seen in both panels is that as
by cyclic voltammetry. The constructed POM assemblies were
then characterized through the employment of electrochemical
techniques, UV−vis spectroscopy, AFM, and XPS.
3.1. Solution Electrochemistry of P8W48Fe16. Figure 2
represents the cyclic voltammograms obtained at a GCE for the
Figure 2. pH effect of POM redox activity in solution. Cyclic
voltammograms were recorded at GCE for a LiK- P8W48Fe16 solution
(0.04 mM) in pH 2 buffer (A) and pH 4.5 buffer (B); scan rate
10 mV s−1.
redox chemistry of P8W48Fe16 at both pH 2 and pH 4.5. The
reduction peak observed at −0.21 V in curve A (pH 2)
represents the simultaneous one-electron reduction of the 16
iron(III) centers in P8W48Fe16.53 The corresponding reox-
idation peak for these iron centers appears at +0.588 V. The
next two reduction processes (I and II), which are observed at
−0.37 V and −0.54 V, represent two consecutive eight-electron
redox-processes associated with the POM’s tungsten-oxo
(W−O) framework. It is well-known that POMs exhibit pH-
dependent redox processes both in solution and when surface
immobilized.54−56 McCormac et al. have previously shown that
the metal ion-substituted Wells−Dawson POMs possess pH-
dependent single and multiple electron redox processes.8 The
cathodic shift in the measured redox potentials for these Figure 3. Chemical switching of P8W48Fe16−MB LBL film.
Consecutive cyclic voltammograms of LBL film recorded after eight
tungsten-oxo processes with an increase in pH (curve B at
“POM” deposition steps (A) and after eight “MB” deposition steps
Figure 2) reveals the involvement of protons in the (B). 0.5 M Li2SO4, 0.5 M H2SO4, pH 2, scan rate 10 mVs−1. (C) The
aforementioned redox processes. The potential shifts exceed dependences of peak charges (□ - MB reduction peak; ■ - W−O II
the value of 59 mV/pH, which is typical for the POMs redox oxidation peak) on the number of layers. Inset: LBL film growth onto
processes with the same numbers of both protons and electrons PDDA-modified ITO slides monitored by UV−vis spectroscopy after
being involved.57 The redox behavior of the dye, MB, at pH 2 is MB steps of different assembly numbers.
5482 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
the number of layers deposited onto the electrode surface P 8 W 48 Fe 16 , the E 1/2 values for the first and second
increases the currents associated with the redox chemistry of W−O-based redox processes shift from −0.313 V and −0.512 V,
both the P8W48Fe16 and MB moieties, thus indicating the in solution, to −0.262 V (ΔEp 10 mV) and −0.543 V (ΔEp
successful inclusion of both these moieties into the multilayer 7 mV), respectively in the immobilized state with an outer POM
system. In addition, when the MB layer is the outer layer the layer. When the outer layer is cationic in nature, these two W−O
redox activity for the POMs 16 Fe(III) centers is not apparent. redox processes shift to −0.300 V (ΔEp 86 mV) and −0.553 V
The reason for this remains unclear at present. Figure 3C then (ΔEp 19 mV). It can be seen in the latter case that the peak-to-
represents the dependence of peak charges for the POM’s peak separations have increased in value, which would reflect the
W−O second oxidation wave and for the MB reduction wave difficulty of charge balancing protons from the contacting electrolyte
on the numbers of deposited layers within the LBL assembly. passing into the film through the outer cationic layer upon redox
Figure 3C shows a decrease in the redox peak currents and switching through the POM’s W−O-based processes. A similar
associated charge related to the second W−O redox process for control of electrochemical properties of LBL film-modified
the P8W48Fe16 POM after the deposition of each MB cationic electrodes due to the nature of the terminal layer has been
layer, which is also accompanied by an increase in the capacitive observed with Pt nanoparticle-based deposits.51
currents. This commences after the deposition of the fifth Figure 4A,B illustrates the resulting cyclic voltammograms
cationic MB layer and becomes more pronounced as more for the P8W48Fe16−MB multilayer films as a function of scan
layers are deposited on the LBL assembly. However, for the
MB reduction wave, as the numbers of layers is increased, there
is a gradual increase in the redox process’s charge with no
associated decrease upon the addition of each POM layer.
However by the deposition of the 10th POM layer and
thereafter, there is a continual decrease in the associated charge
for the MB reduction process upon the addition of each POM
layer.
The role of both anionic and cationic species moving into/
out of the film, from the contacting electrolyte, upon redox
switching of the film, must play a role in this observed film
behavior. In addition, the presence of an outer cationic MB
layer must hinder the passage of protons from the supporting
electrolyte into the film upon the redox switching of the film
through the POM’s W−O based processes. In addition, the
difference in the porosity of the film when there is either an
outer anionic or cationic layer would play a role in the observed
electrochemical behavior of the film. There is a continuous
growth in the multilayer’s surface coverage from 0.003 nmol
cm2 for the second POM layer to 0.3 nmol cm2 for 16th POM
layer, and from 0.015 nmol cm2 for the first MB layer to 0.6
nmol cm2 for the 15th MB layer.
The growth of the LBL films was also monitored by UV−vis
spectroscopy as shown in Figure 3D. MB exhibits a blue shift
(Soret band) after deposition by changing the λmax from 664
nm for the monomer to 605 nm for eight layers of MB.
Generally, dyes form aggregates with anionic species while
being modified onto substrates. According to the literature, MB
belongs to phenothiazine dyes, and the H-aggregates of
phenothiazine systems are formed through π−π staking, with
other factors also influencing the formation, such as molecular Figure 4. Scan rate studies of P8W48Fe16−MB LBL films composed of
structures and templating reagents.59 As previously observed,59 16 layers with an outer POM layer (A) and composed of 17 layers
it is proposed here that H-aggregates between the MB and with an outer MB layer (B). Inset: the scan rate dependences for the
POM are formed because of the rigidity of the POM, which first (I) W−O anodic wave at the LBL film with an outer POM layer
could fit inside the MB packing for stability onto the substrate. (□) and an outer MB layer (■). 0.5 M Li2SO4, 0.5 M H2SO4, pH 2.
When the LBL film is exposed to sun light for 48 h, no changes
in the absorbance spectrum are observed; in addition, exposure rate, for films with an outer POM or MB layer, respectively.
of the LBL film-modified ITO slide to temperatures up to 433K The redox processes associated with the POM’s second W−O
did not lead to spectral changes, thus showing the inherent redox process and MB showed thin layer behavior for up to 1
stability of the P8W48Fe16−MB multilayer films. V/s when the outer layer was cationic in nature. However, the
Upon immobilization within the multilayer assembly, there second W−O redox process showed thin layer behavior up to
were subtle changes in the redox behavior of both the MB and 100 mV/s when the outer layer was anionic in nature (Inset of
P8W48Fe16 moieties as compared to their solution behavior. Figure 4B).
The E1/2 value for the MB redox process shifts from +0.133 V The stability of LBL films was investigated by redox
in solution to +0.091 V and +0.103 V within the multilayer switching the film through the various redox processes and
assembly when the outer layer is either anionic or cationic in monitoring the associated change in redox peak currents at pH 2.
nature, respectively. In terms of the redox activity for For multilayers composed of an outer P8W48Fe16 layer, the
5483 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
current associated with the second W−O (II) POM-based Fe(CN)63−/Fe(CN)64− redox system, that is, +0.27 V. Figure 6A
redox process increased by approximately 50% after 500 redox shows the impedance spectra, as a Nyquist plot, obtained
cycles, which could indicate film saturation with ions injected during the various stages of the deposition of the multilayer
into the film upon cycling. However, the redox activity
associated with the POM’s Fe(III) centers was found to be
unstable upon redox cycling at pH 2.
3.2.1. Effect of Solution pH. It is well known that the redox
processes associated with the W−O frameworks of POMs are
pH-dependent in nature, either in the solution or in the
immobilized state.3,6,8,12 Figure 5 exhibits the effect of the
Figure 5. pH effect upon layer’s redox activity. Cyclic voltammograms
were recorded at an electrode modified with an LBL film composed of
16 layers with an outer POM layer (A,B) and composed of 17 layers
with an outer MB layer (C,D) in pH 2 (A,C; 0.5 M Li2SO4, 0.5 M
H2SO4) and in pH 5 (B,D; 1 M CH3COOLi, 1 M CH3COOH)
buffers; scan rate 10 mVs−1.
solution pH upon the redox activity of the P8W48Fe16−MB
multilayer films when the outer layer is anionic (Figures 5A,B)
or when it is cationic (Figures 5C and D) in nature. What is
observed is that, as the pH is made more alkaline, there is a
cathodic shift in the E1/2 values for the POM’s W−O processes
and the MB redox processes. In addition, for layers composed
of an outer POM layer there appears to be no shift in the 16
electron oxidation of the Fe (II) centers within the POM. The
shifts observed for the POM’s second W−O redox process Figure 6. Chemical switching of the P8W48Fe16−MB LBL film
were 78.8(±3.4) and 76(±2.54) mV pH−1 for films composed monitored by impedance spectroscopy. (A) Nyquist plot of impedance
of an outer POM or MB layer, respectively. The shifts observed spectra of electrode modified with P8W48Fe16−MB LBL films (■1 -
for the bielectronic redox process of MB were 84.3(±3.97) and spectrum of blank GCE; □2 - spectrum of PDDA-modified electrode;
71.4(±2.99) mV pH−1, respectively. ▲3, ●5 and ■7 - spectra of modified electrode after POM deposition
3.2.2. Electrochemical Impedance Spectroscopy. EIS steps; ○4, □6, and △8 - spectra of modified electrode after MB
studies have been carried at the various layer depositions deposition steps) in ferro/ferricyanide solution (10 mM K3[Fe(CN)6],
10 mM K4[Fe(CN)6], 0.1 M KCl); 10 mV amplitude, 230 mV
during the growth of the P8W48Fe16−MB multilayer films. As potential of measurement. (B,C,D) The dependencies of fitted values
detailed in the Experimental Section, the redox probe, of Randles circuit elements (double layer capacitance, charge transfer
Potassium ferri/ferrocyanide, was employed, with the applied resistance and Warburg impedance) on the numbers of assembly
experimental potential being set at the formal potential of the layers.
5484 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
assembly. What is observed is that the values of the charge
transfer resistance RCT, which can be roughly estimated as the
diameter of a semicircle at the kinetically controlled region,
repeatedly increase after the deposition of each of the
P8W48Fe16 anionic layers but decrease after the deposition of
the cationic MB layers.
The interpretation of impedance data has been carried out
through the employment of an equivalent Randles circuit,
which consists of double-layer capacitance in series with a
solution resistance and in parallel with a diffusional branch, i.e.,
Warburg impedance and charge transfer resistance. The
constant phase element was introduced instead of the double-
layer capacitance, which illustrates the nonuniform distribution
of the capacitance over the electrode surface. Fitting of the
resulting data revealed the switching behavior of the double
layer capacitance Cdl (Figure 6B). It can be seen that the
modification of the blank GCE with the first layer of PDDA led
to a 60% decrease in Cdl. The adsorption of P8W48Fe16
polyanion at an even layer number led to subsequent increases Figure 7. Permeability of films by anionic redox probe. (A) LBL film
in both Cdl and RCT (Figure 6C). This probably reflects the composed of eight layers with an outer POM layer; (B) LBL film
more compact “less porous” nature of the highly charged composed of nine layers with an outer MB layer; (C) LBL film
P8W48Fe16 POM layers. composed of 16 layers with an outer POM layer; (D) LBL film of 17
The decrease of RCT values after the deposition of the layers with an outer MB layer. Cyclic voltammograms were recorded at
pH 2 buffer (0.5 M Li2SO4, 0.5 M H2SO4) before (thin line) and after
cationic MB layers can be a result of attractive interactions (thick line) addition of 1 mM K3[Fe(CN)6]. Dashed line -
between the positively charged MB molecules at the outer layer voltammogram at blank GCE.
of the LBL film-modified electrode and negatively charged
redox probe molecules, which enhance the probe diffusion absence of redox chemistry for [Fe(CN)6]3−/4−, thus indicating
through the film to the underlying electrode surface. The effect the lack of film porosity at this film thickness.
of the increase in Cdl diminishes for higher layer numbers, 3.2.4. AFM Imaging and XPS. AFM imaging of the
which illustrates the building of the LBL film and the lower P8W48Fe16−MB LBL film at ITO glass slides was performed
impact on the double layer at electrode/solution interface. The to find out the topography of the deposits. Figure 8 presents
change in RCT between the two deposition steps is enhanced the AFM images of a blank ITO slide (Figure 8A) and slides
with increasing layer number, which influences the redox probe after the first (PDDA, Figure 8B), second (first POM step,
diffusion through the film. The Warburg impedance (Figure Figure 8C), and 17th layers (Figure 8D) of LBL assembly.
6D), which represents the thickening of diffusion through the Root-mean-square surface roughness parameters were 22.6,
film, increases sharply after the first layer deposition and then 23.6, 10.4, and 4.5 nm, respectively. Larger features from the
sequentially decreases. This effect is probably due to the topography of the ITO substrate were also seen in the images
enhancement of active surface available for charge transfer obtained from glass slide modified with PDDA, along with a
from/to the redox probe. In terms of impedance measure- more globular structure suggestive of a polymer film. Further
ments, the film exhibited good stability. changes in surface topography were observed for the first POM
3.2.3. Film Permeability. The porosity of the P8W48Fe16− layer and multilayer films. The deposition of first POM layer
MB multilayer films was investigated by studying the effect of a led to sufficient decrease of surface roughness. Increasing the
redox probe, namely, [Fe(CN)6]3−/4−, upon the voltammetry of number of deposited layers resulted in further reduced film
the multilayer assembly. The fate of the probe when in contact defects. The topography of samples B and D featured globular
with the multilayer assembly can be one of the following: the structures. Little phase contrast was seen for sample D, which
probe can diffuse through the assembly and undergo reaction at illustrates a homogeneous surface of deposit within the areas of
the underlying electrode surface, or can undergo redox reaction interest imaged. The values of surface roughness reported
at the film/solution interface by mediated electron transfer by previously for the similar systems were in comparison to what
redox sites present within the LBL film.6 The anionic probe, we got here. Quartz slides deposited with the layer by layer
[Fe(CN)6]3−/4−, exhibits a monoelectronic redox process with assembly of [Eu(SiW10VO39)2]15− and polyethyleneimine
an E1/2 of +0.21 V at pH 2. Figure 7 presents the voltammetric displayed a surface roughness of 2.4 nm.58 Also the Fluorine-
responses of the ferri/ferrocyanide couple obtained at a blank doped tin oxide thin films deposited by chemical vapor
GCE (Figure 7A, dashed line) and at electrodes modified with deposition showed surface roughness values between 5 and
two LBL films of different thicknesses. For the two films 35 nm.59 XPS analysis of the P8W48Fe16−MB LBL film showed
composed of four bilayers, with either an outer POM (Figure the presence of N (4.8%), C (50.1%), O (33.5%), W (7.6%),
7A) or an outer MB (Figure 7B), a degree of film porosity is and Fe (1%).
apparent as the [Fe(CN)6]3−/4− is able to diffuse through the 3.2.5. Preliminary Electrocatalytic Properties of LBL Films.
multilayer assembly and react at the underlying electrode POMs have previously been employed for the reduction of
surface albeit with reduced peak currents as opposed to the hydrogen peroxide.3,60,61 The ability of the P8W48Fe16−MB
[Fe(CN)6]3−/4− at the bare electrode surface. However, as seen LBL films, when the outer layer is either anionic or cationic, to
in Figure 7C,D, for multilayer films composed of eight bilayers, electrocatalytically reduce hydrogen peroxide has been
with either an outer POM or cationic MB layer, there is an investigated. Figure 9A represents the voltammetric responses
5485 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
Figure 8. AFM images of ITO glass slides during different stages of layer construction. (A) blank slide, (B) slide modified with initial PDDA layer,
(C) slide after subsequent first POM deposition step and (D) slide after 16th POM layer deposition.
outer layer of the multilayer assembly is the MB cationic
moiety, then no electrocatalytic effect is observed, as seen in
Figure 9B. These preliminary results support our findings
detailed in previous sections, where the nature of the outer
layer has an effect on the multilayer’s properties.
■ CONCLUSIONS
Stable and reproducible multilayer films composed of the iron-
substituted POM Li4K16[P8W48O184Fe16(OH)28-
(H2O)4]·66H2O·2KCl and MB have been deposited on carbon
and ITO electrode surfaces through the employment of the
LBL technique. It was found that such layers exhibited the
expected pH-dependent redox activity associated with both the
Figure 9. Cyclic voltammograms obtained for electrode-modified POM and MB species. The films were found to exhibit thin
P8W48Fe16−MB films upon the addition of hydrogen peroxide. layer behavior up to 100 mV s−1 when the outer layer was
P8W48Fe16−MB films composed of 12 layers with an outer POM layer anionic in nature, and 1 V s−1 when the outer layer was cationic.
(A) and 13 layers with an outer MB layer (B) in the absence (solid Through the employment of AC impedance and cyclic
lines) and after addition of hydrogen peroxide (0.2 mM and 0.8 mM,
voltammetry, it was found that the redox switching and
dashed lines). 1 M H2SO4; scan rate 10 mV/s.
permeability of the constructed layers was dependent on both
of P8W48Fe16−MB LBL film-modified electrodes composed of the layer thickness and the nature of the outermost layer.
Preliminary investigations showed that the films exhibited the
eight bilayers with an outer POM layer in the absence and
ability to electrocatalytically reduce hydrogen peroxide only
presence of both 0.2 and 0.8 mM hydrogen peroxide. What is
when the outer layer was anionic in nature.
■
clearly observed is an increase in the reduction currents
associated with the POM’s tungsten-oxo redox processes upon
successive additions of H2O2. This indicates that it is the AUTHOR INFORMATION
multiply reduced form of the POM, which catalyzes the Corresponding Author
reduction of the added H2O2. The measured catalytic currents *Fax: +353 42 933 1163; Tel: +353 42 937 4579; E-mail: tim.
were found to be linear up to 3 mM. Interestingly when the mccormac@dkit.ie.
5486 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
Notes (21) Mizuno, N.; Misono, M. Heteropolyacid catalysts. Curr. Opin.
The authors declare no competing financial interest. Solid State Mater. Sci. 1997, 2 (1), 84−89.
■
(22) Rong, C. Y.; Anson, F. C. Spontaneous adsorption of
REFERENCES heteropolytungstates and heteropolymolybdates on the surfaces of
solid electrodes and the electrocatalytic activity of the adsorbed anions.
(1) Pope, M. T. Heteropoly and Isopoly Oxometalates; Springer Verlag:
Inorg. Chim. Acta 1996, 242 (1−2), 11−16.
New York, 1983.
(23) Shimidzu, T.; Ohtani, A.; Aiba, M.; Honda, K. Electrochromism
(2) Pope, M. T.; Muller, A. Polyoxometalate chemistry - An old field
with new dimensions in several disciplines. Angew. Chem., Int. Ed. Engl. of a conducting polypyrrole−phosphotungstate composite electrode.
1991, 30 (1), 34−48. J. Chem. Soc., Faraday Trans. 1 1988, 84, 3941−3949.
(3) Bi, L. H.; Foster, K.; McCormac, T.; Dempsey, E. Preparation of (24) Wang, B. X.; Dong, S. J. Electrochemical study of isopoly-
multilayer films containing a crown heteropolyanion and an osmium oxometallates and heteropoly-oxometallates film modified micro-
functionalised pyrrole monomer. J. Electroanal. Chem. 2007, 605 (1), electrodes. 3. Further study of electrode process of isopolymolybdic
24−30. anion doped in polyaniline film. Electrochim. Acta 1993, 38 (7), 1029−
(4) Borras-Almenar, J. J.; Coronado, E.; Muller, A.; Pope, M. T. 1035.
Polyoxometalates Molecular Science; Kluwer: Dordrecht, The Nether- (25) Ammam, M.; Keita, B.; Nadjo, L.; Mbomekalle, I. M.; Fransaer,
lands, 2004. J. Attempts to immobilize catalytically active substituted-heteropoly-
(5) Casan-Pastor, N.; Gomez-Romero, P. Polyoxometalates: From tungstates in multilayer film of charged polyelectrolyte poly(allylamine
inorganic chemistry to materials science. Front. Biosci. 2004, 9, 1759− hydrochloride). J. Electroanal. Chem. 2010, 645 (2), 65−73.
1770. (26) Wang, Y. H.; Hu, C. W. Layer-by-layer self-assembly of dye−
(6) Fay, N.; Dempsey, E.; McCormac, T. Assembly, electrochemical polyoxometalate multilayer composite films and their fluorescent
characterisation and electrocatalytic ability of multilayer films based on properties. Thin Solid Films 2005, 476 (1), 84−91.
[Fe(bpy)3]2+, and the Dawson heteropolyanion, [P2W18O62]6−. (27) Iler, R. K. Multilayers of colloidal particles. J. Colloid Interface Sci.
J. Electroanal. Chem. 2005, 574 (2), 359−366. 1966, 21 (6), 569−594.
(7) Hill, C. L. Polyoxometalates: Reactivity. In Comprehensive (28) Decher, G.; Hong, J. D.; Schmitt, J. Buildup of ultrathin
Coordination Chemistry II; Webb, A. G., Ed.; Elsevier: New York, multilayer films by a self-assembly process: III. Consecutively
2003; Vol. 4, pp 679−759. alternating adsorption of anionic and cationic polyelectrolytes on
(8) McCormac, T.; Fabre, B.; Bidan, G. Role of pH and the transition charged surfaces. Thin Solid Films 1992, 210−211 (2), 831−835.
metal for the electrocatalytic reduction of nitrite with transition metal (29) Jiang, M.; Wang, E.; Wang, X. L.; Wu, A. G.; Kang, Z. H.; Lian,
substituted Dawson type heteropolyanions 0.2. J. Electroanal. Chem. S. Y.; Xu, L.; Li, Z. Self-assembly of lacunary Dawson type
1997, 427 (1−2), 155−159. polyoxometalates and poly(allylamine hydrochloride) multilayer
(9) Pope, M. T. Polyoxo anions: Synthesis and structure. films: photoluminescent and electrochemical behavior. Appl. Surf. Sci.
Comprehensive Coordination Chemistry II 2003, 4, 635−678. 2005, 242 (1−2), 199−206.
(10) Pope, M. T.; Muller, A. Polyoxometalates Chemistry: From (30) Zhai, S. Y.; Gong, S. Y.; Jiang, J. U.; Dong, S. J.; Li, J. H.
Topology via Self Assembly to Applicaation.; Kluwer Academic Assembly of multilayer films containing iron(III)-substituted Dawson-
Publishers: Dordrecht, The Netherlands, 2001; pp 1−467. type heteropolyanions and its electrocatalytic properties: Cyclic
(11) Yamase, T.; Pope, M. T. Polyoxometalate Chemistry for Nano- voltammetry, electrochemical impedance spectroscopy and UV−vis
Composite Design; Kluwer Academic/Plenum Publishers: New York, spectrometry. Anal. Chim. Acta 2003, 486 (1), 85−92.
2002; pp 1−227. (31) Zhang, X.; Chen, H.; Zhang, H. Y. Layer-by-layer assembly:
(12) Zynek, M.; Serantoni, M.; Beloshapkin, S.; Dempsey, E.; From conventional to unconventional methods. Chem. Commun. 2007,
McCormac, T. Electrochemical and surface properties of multilayer No. 14, 1395−1405.
films based on a Ru2+ metallodendrimer and the mixed addenda (32) Gao, S. Y.; Cao, R.; Li, X. Self-assembly of Keggin-type
Dawson heteropolyanion. Electroanalysis 2007, 19 (6), 681−689. tungstoborate-based multilayer films and their electrochemical
(13) Abrantes, L. M.; Cordas, C. M.; Vieil, E. EQCM study of behavior. Thin Solid Films 2006, 500 (1−2), 283−288.
polypyrrole modified electrodes doped with Keggin-type hetero- (33) Gao, S. Y.; Li, T. H.; Li, X.; Cao, R. Electrochemical behavior
polyanion for cation detection. Electrochim. Acta 2002, 47 (9), 1481− and multilayer films of the sandwich-type polyoxotungstate complex
1487.
{K10Co4(H2O)2(PW9O34)2}. Mater. Lett. 2006, 60 (29−30), 3622−
(14) Averbach, A. Stabilization of Photochromic Copy. U.S. Patent
3626.
3,623,866, 1971.
(34) Liu, S. Q.; Volkmer, D.; Kurth, D. G. Functional
(15) Bao, Y. Y.; Bi, L. H.; Wu, L. X.; Mal, S. S.; Kortz, U. Preparation
polyoxometalate thin films via electrostatic layer-by-layer self-assembly.
and Characterization of Langmuir−Blodgett Films of Wheel-Shaped
Cu-20 Tungstophosphate and DODA by Two Different Strategies. J. Cluster Sci. 2003, 14 (3), 405−419.
Langmuir 2009, 25 (22), 13000−13006. (35) Cheng, L.; Cox, J. A. Nanocomposite multilayer film of a
(16) Barth, M.; Lapkowski, M.; Lefrant, S. Electrochemical behaviour ruthenium metallodendrimer and a Dawson-type polyoxometalate as a
of polyaniline films doped with heteropolyanions of Keggin structure. bifunctional electrocatalyst. Chem. Mater. 2002, 14 (1), 6−+.
Electrochim. Acta 1999, 44 (12), 2117−2123. (36) Cheng, L.; Dong, S. Comparative studies on electrochemical
(17) Bidan, G.; Genies, E. M.; Lapkowski, M. One-step electro- behavior and electrocatalytic properties of heteropolyanion-containing
chemical immobilization of Keggin-type heteropolyanions in poly(3- multilayer films prepared by two methods. J. Electroanal. Chem. 2000,
methylthiophene) film at an electrode surface - Electrochemical and 481 (2), 168−176.
electrocatalytic properties. Synth. Met. 1989, 31 (3), 327−334. (37) Kuhn, A.; Anson, F. C. Adsorption of monolayers of
(18) Haussling, L.; Ringsdorf, H.; Schmitt, F. J.; Knoll, W. Biotin- P2Mo18O626− and deposition of multiple layers of Os(bpy)32+−
functionalized self-assembled monolayers on gold: Surface-plasmon P2Mo18O626− on electrode surfaces. Langmuir 1996, 12 (22), 5481−
optical studies of specific recognition reactions. Langmuir 1991, 7 (9), 5488.
1837−1840. (38) Ma, H. Y.; Dong, T.; Wang, F. P.; Zhang, W.; Zhou, B. B. A
(19) Keita, B.; Bouaziz, D.; Nadjo, L. Strategies for entrapping multifunctional organic−inorganic multilayer film based on tris(1,10-
oxometalates in polymeric matrices - Example of polyaniline as the phenanthroline)ruthenium and polyoxometalate. Electrochim. Acta
matrix. J. Electroanal. Chem. 1988, 255 (1−2), 303−313. 2006, 51 (23), 4965−4970.
(20) Lapkowski, M.; Bidan, G.; Fournier, M. Synthesis of polypyrrole (39) Feng, Y. H.; Peng, J.; Han, Z. G.; Ma, H. Y. Fabrication of
and polythiophene in aquaoues solutions of Keggin-type structure photosensitive multilayer films based on polyoxometalate and
heteropolyanions. Synth. Met. 1991, 41 (1−2), 407−410. diazoresin. J. Colloid Interface Sci. 2005, 286 (2), 589−595.
5487 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488Langmuir Article
(40) Bi, L. H.; Wang, H. Y.; Shen, Y.; Wang, E. K.; Dong, S. J. (58) Ma, H.; Peng, J.; Han, Z.; Feng, Y.; Wang, E. Preparation and
Multifunctional organic−inorganic multilayer films of tris(2,2′- characterization of luminescent nanocomposite film containing
bipyridine)ruthenium and decatungstate. Electrochem. Commun. polyoxometalate. Thin Solid Films 2004, 446, 161−166.
2003, 5 (11), 913−918. (59) Dahou, F. Z.; Cattin, L.; Garnier, J.; Ouerfelli, J.; Morsli, M.;
(41) Liu, J. Y.; Cheng, L.; Liu, B. F.; Dong, S. J. Multilayer assemblies Louarn, G.; Bouteville, A.; Khellil, A.; Beréde, J. C. Influence of anode
of tungstodiphosphate anions on 1,7-diaminoheptane modified glassy roughness and buffer layer nature on organic solar cells performance.
carbon electrode and the electrocatalytic reduction to iodate. Thin Solid Films 2010, 518, 6117−6122.
Electroanalysis 2001, 13 (12), 993−998. (60) Ernst, A. Z.; Zoladek, S.; Wiaderek, K.; Cox, J. A.; Kolary-
(42) Wang, L.; Li, J.; Wang, E. B.; Xu, L.; Peng, J.; Li, Z. Preparation Zurowska, A.; Mieunikowski, K.; Kulesza, P. J. Network films of
and characterization of ultrathin multilayer films based on poly- conducting polymer-linked polyoxometalate-modified gold nano-
oxometalate Mo8V2O28·7H2O. Mater. Lett. 2004, 58 (14), 2027−2031. particles: Preparation and electrochemical characterization. Electro-
(43) Zhang, Y. Q.; Wang, K. Z.; Gao, L. H. Electrostatically self- chim. Acta 2008, 53 (11), 3924−3931.
assembled multilayer film formed by a novel ruthenium(II) complex (61) Toth, J. E.; Melton, J. D.; Cabelli, D.; Bielski, B. H. J.; Anson, F. C.
and Nd(SiW9Mo2O39)213−. Colloids Surf., A: Physicochem. Eng. Aspects Electrochemistry and redox chemistry of H2OFeIIISiW11O395− in the
2005, 257−58, 391−394. presence of H2O2 and OH. Inorg. Chem. 1990, 29 (10), 1952−1957.
(44) Cheng, L.; Niu, L.; Gong, J.; Dong, S. J. Electrochemical growth
and characterization of polyoxometalate-containing monolayers and
multilayers on alkanethiol monolayers self-assembled on gold
electrodes. Chem. Mater. 1999, 11 (6), 1465−1475.
(45) Bourbon, S.; Gao, M. Y.; Kirstein, S. Electroluminescence of
self-assembled films of poly(p-phenylene vinylene) and J-aggregates.
Synth. Met. 1999, 101 (1−3), 152−153.
(46) Fukumoto, H.; Yonezawa, Y. Layer-by-layer self-assembly of
polyelectrolyte and water soluble cyanine dye. Thin Solid Films 1998,
327, 748−751.
(47) Kaneko, F.; Kato, T.; Baba, A.; Shinbo, K.; Kato, K.; Advincula,
R. C. Photo-induced fabrication of surface relief gratings in alternate
self-assembled films containing azo dye and alignments of LC
molecules. Colloids and Surfaces a-Physicochemical and Engineering
Aspects 2002, 198, 805−810.
(48) Lodi, A.; Ponterini, G. J-aggregation of an anionic
oxacarbocyanine in electrostatically self-assembled multilayers. Thin
Solid Films 2006, 496 (2), 585−594.
(49) Nicol, E.; Moussa, A.; Habib-Jiwan, J. L.; Jonas, A. M. Layer-by-
layer self-assembly of polyelectrolyte and the divalent salt of
fluorescein. J. Photochem. Photobiol. A: Chem. 2004, 167 (1), 31−35.
(50) Gao, S. Y.; Cao, R.; Yang, C. P. Dye-polyoxometalate composite
films: Self-assembly, thermal and photochemical properties. J. Colloid
Interface Sci. 2008, 324 (1−2), 156−166.
(51) Horswell, S. L.; O’Neil, I. A.; Schiffrin, D. J. Kinetics of electron
transfer at Pt nanostructured film electrodes. J. Phys. Chem. B 2003,
107, 4844−4854.
(52) Sartorel, A.; Carraro, M.; Scorrano, G.; Bassil, B. S.; Dickman,
M. H.; Keita, B.; Nadjo, L.; Kortz, U.; Bonchio, M. Iron-substituted
polyoxotungstates as inorganic synzymes: Evidence for a biomimetic
pathway in the catalytic oxygenation of catechols. Chem.Eur. J. 2009,
15 (32), 7854−7858.
(53) Mal, S. S.; Dickman, M. H.; Kortz, U.; Todea, A. M.; Merca, A.;
Bogge, H.; Glaser, T.; Muller, A.; Nellutla, S.; Kaur, N.; van Tol, J.;
Dalal, N. S.; Keita, B.; Nadjo, L. Nucleation process in the cavity of a
48-tungstophosphate wheel resulting in a 16-metal-centre iron oxide
nanocluster. Chem.Eur. J. 2008, 14 (4), 1186−1195.
(54) Ruhlmann, L.; Genet, G. Wells−Dawson-derived tetrameric
complexes {K28H8 P2W15Ti3O60.5}4 electrochemical behaviour and
electrocatalytic reduction of nitrite and of nitric oxide. J. Electroanal.
Chem. 2004, 568 (1−2), 315−321.
(55) Wang, C. L.; Liu, S. X.; Sun, C. Y.; Xie, L. H.; Ren, Y. H.; Liang,
D. D.; Cheng, H. Y. Bimetals substituted germanotungstate complexes
with open Wells-Dawson structure: Synthesis, structure, and electro-
chemical behavior of {M(H 2 O)} (μ-H 2 O) 2 K{M(H 2 O) 4 }-
(Ge2W18O66)11− (M = Co, Ni, Mn). J. Mol. Struct. 2007, 841 (1−3),
88−95.
(56) Wang, P.; Li, Y. F. Electrochemical and electrocatalytic
properties of polypyrrole film doped with heteropolyanions.
J. Electroanal. Chem. 1996, 408 (1−2), 77−81.
(57) McCormac, T.; Fabre, B.; Bidan, G.; Part, I. A comparative
electrochemical study of transition metal substituted Dawson type
heteropolyanions. J. Electroanal. Chem. 1997, 425, 49−54.
5488 dx.doi.org/10.1021/la3004068 | Langmuir 2012, 28, 5480−5488You can also read