Approaches to control π-π-interactions and solubility of heteroatomic redox polymers in rechargeable Li/Organic batteries - Verena Perner, Fabian ...
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Approaches to control π-π-interactions and solubility of heteroatomic redox polymers in rechargeable Li/Organic batteries Verena Perner, Fabian Otteny, Birgit Esser, Peter Bieker, Martin Winter, Martin Kolek Advanced Battery Power 2021
Previous work
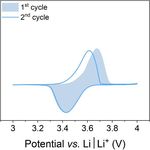
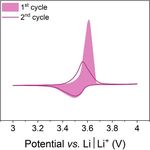
charge
A B C
Poly(3-vinyl-N-methylphenothiazine)
PVMPT discharge
• Charge stabilization via π-π interactions
• High cycling stability & rate capability
• Specific capacity: 56 mAh g−1 (theoretical: 112 mAh g−1)
• Soluble in electrolyte
• Interplay of π-π interactions and dissolution
March 31, 2021 Kolek M. et al, Energy Environ. Sci., 2017, 10, 2334 – 2341. WE: PVMPT (50 wt.%), Super C65, PVdF 2
Kolek M. et al, Chem. Mater., 2018, 30, 6307 – 6317. CE, RE: Li foil
Otteny F. et al, Adv. Energy Mater., 2018, 8, 1802151. Electrolyte: 1 M LiPF6 / EC : DMC (1 : 1)Approaches to control π-π interactions & solubility
Solubility
Tailoring of π-π interactions
Electrolytes
soluble insoluble
Spacing design Variation of heteroatom
31 March 2021 Perner V. et al, ACS Appl. Mater. Interfaces 2021, 13, 12442–12453. 3
Perner V. et al, ACS Sustainable Chem. Eng. 2020, 8, 238−247.
Studer G. et al, ChemSusChem 2020, 13, 2232 –2238.Approaches to influence π-π interactions
intramolecular No intramolecular π-π interactions
Poly(3-vinyl-N-methylphenothiazine)
alternating N-methylmaleimide
P(VMPT-alt-MM)
intermolecular
No intra- or intermolecular π-π interactions
Poly(3-vinyl-N-o-tolylphenothiazine) Poly(3-vinyl-N-mesitylphenothiazine)
PV-o-TolPT PVMesPT
31 March 2021 4Influencing π-π interactions
Behaviour of polymers during oxidation
PV-o-TolPT PVMesPT P(VMPT-alt-MM) • Band for the dimer (oxidation state
C) of PVMPT strongly decreased
for PVMesPT and PV-o-TolPT
• Only bands for neutral polymer #
(≈ 240 nm) and radical cation §
(≈ 520 nm) of MPT observed
• Dissolution and re-deposition of
PV-o-TolPT PVMesPT P(VMPT-alt-MM) PVMPT during cycling
• Strong interaction of MPT units is
assumed to be important for re-
deposition and increase of capacity
• Solvation of radicals is favored →
re-deposition hindered by
decreased interactions
31 March 2021 Kolek M. et al, Chem. Mater., 2018, 30, 6307 – 6317. WE: Polymer (50 wt.%), Super C65, PVdF 5
Morishima Y. et al, J. Polym. Sci. Polym., Lett. Ed. 1985, 23, 651 – 653. CE, RE: Li foil
Electrolyte: 1 M LiPF6 / EC : DMC (1 : 1)Influencing π-π interactions
Substitution of heteroatom
• Cycling occurres between the neutral and
fully oxidized state (A and C)
• Reduced cycling stability, but higher rate
capability due to absence of π-π interactions
• Planarization of phenoxazine units
Poly(3-vinyl-N-methylphenoxazine)
PVMPO
March 31, 2021 Perner, V. et al, ACS Sustainable Chem. Eng. 2020, 8, 238-247. WE: PVMPO (50 wt.%), Super C65, PVdF 6
CE, RE: Li foil
Electrolyte: 1 M LiPF6 / EC : DMC (1 : 1)Influence of electrolyte on solubility
Variation of solvents
EC:DMC (1:1) EC:DMC (3:7) EC:EMC (1:1) EC:EMC (3:7)
• The choice of electrolyte has strong influence on the redox behavior
• Lowest solubility and therefore highest reversibility observed for optimized electrolyte formulation
• Decrease of dielectric constant from 33.6 to 18.5
31 March 2021 Perner V. et al, ACS Appl. Mater. Interfaces 2021, 13, 12442–12453. WE: PVMPT (50 wt.%), Super C65, PVdF 7
CE, RE: Li foil
Electrolyte: 1 M LiPF6 in various solventsSummary & Conclusion
• Variation of components in electrolyte has positive impact on cycling behavior
• Intramolecular π-π interactions between PVMPT units assumed
• Strong interaction of MPT units seems to be important for re-deposition and increase of
capacity
• Change of heteroatom (S → O) has high impact on electrochemical behavior of polymer
The authors wish to thank the German Research Foundation
(DFG) for financial support within HALO(3160392600).
31 March 2021 8You can also read