Corrosion of Borosilicate Glasses - Berliner Glas Gruppe
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Corrosion of Borosilicate Glasses
Constans M. Weber1,*, Jörg Stockmann1,2 and Elisabeth Rosier1
1
Berliner Glas KGaA Herbert Kubatz GmbH & Co., Waldkraiburger Strasse 5, 12347 Berlin
2
BAM Bundesanstalt für Materialforschung und –prüfung, Unter den Eichen 87, 12203 Berlin
* Corresponding author: cweber@berlinerglas.de
Abstract
In contact with aqueous media glass tends to corrode leading to different surface properties. For the precision
application this alteration might cause product failure. Hence precise knowledge of the surface interactions is
essential. We performed an extensive investigation on the behavior of borosilicate glass under corrosive attack in
order to identify critical processes. We identified appropriate investigation methods including sequential chemical
analysis, ATR-IR, ellipsometry and SNMS. These techniques allow to identify the influence of specific production
processes.
Key words: glass corrosion, borosilicate glass, SNMS, ellipsometry, ATR-IR
performed an extensive investigation of the corrosion
1. INTRODUCTION behavior of borosilicate glass, including sequential
chemical analysis, ellipsometry, Attenuated Total
The surface of certain components in the Reflection Infrared spectroscopy (ATR-IR) and
lithography industry needs to be of glassy material Secondary Neutral Mass Spectroscopy (SNMS).
due to the very high requirements with respect to the
surface roughness and flatness. In the case for the 2. EXPERIMENTAL
components produced by Berliner Glas this surface is
an alkaline free borosilicate glass. Due to the very Pieces of bare polished borosilicate glass were
high surface standards it is necessary to know the used for experiments. The samples underwent a chain
behavior of the glass surface during the production of typical processes in order to capture even effects
process. In particular the interaction with aqueous that originate from the combination of processes. The
systems applied while polishing, cleaning, and final processes of the samples are:
etching may alter the surface in an unwanted manner. G101: Reference (no processing)
It is known that ions can diffuse out of the surface G102: Caro’s acid (piranha)
layers of the glass [1, 2]. This leads to different
G103: diluted hydrofluoric acid (HF) dip and rinse in
properties of the relevant top surface from the bulk
sodium carbonate
properties. Common alterations of the surface include
homogeneous gel layers (subsurface zone), gel G104: HF etch
droplets, crystallite and hole formation (see Figure G105: Cr coating and removal with Cr Etch
1). The modifications are present within some tens to G106: Ion Beam Figuring (IBF) and diluted HF dip
hundreds of nanometers of the surface. Therefore we
The glass investigated with SNMS underwent similar
processes but is part of a symmetrical stack of glass
with a ceramic body.
The experimental techniques were chosen in
order to investigate the relevant region of the
Figure 1: Dimensions of typical degradations from left to samples. Figure 2 shows the considerations that lead
right: Homogeneous gel layer, gel droplet formation, to the choice of the mentioned techniques. Surface
formation of crystallites, and holes and craters [2]. sensitive methods like AFM or contact anglemeasurements only account for the topmost surface can capture gel droplets on the surface. Compared to
region (green in Figure 2) whereas bulk methods like other glasses these findings are considered modest.
Figure 2: Schematic representation of the information
depth of surface sensitive (green), bulk sensitive (yellow)
and intermediate techniques (red).
Figure 3: DIC microscopy of the corrosion layer with gel
XRF and UV-Vis spectroscopy are not sensitive to droplets.
the surface.
Microscopy was performed using a Zeiss Axio 3.2. SEQUENTIAL CHEMICAL ANALYSIS
Imager A2 Vario in brightfield, darkfield and DIC Sequential chemical analysis shows non-bulk
(Differential Interference Contrast) illumination. properties only in the first step of the investigation.
Sequential chemical analysis includes short dips This again indicates a stable system compared to
in diluted hydrofluoric acid, precision weighing of other glasses, still an accumulation of barium ions
the sample and chemical analysis of the applied acid along the process chain can be observed.
[3]. The investigated depth is determined by the
number of steps and the etch time. 3.3. ATR-IR
Ellipsometry was performed using Woolam M- The obtained spectra (see Figure 4) show
2000 (mapping ellipsometry), Accurion EP3 SE significant peaks in the region of 600 to 4000 cm-1.
(imaging ellipsometry) and Sentech SE-900 The individual peak can be allocated to specific
(infrared) ellipsometers. This technique measures the vibrations in the glass [4]. The peaks at 2300 cm-1
dielectric properties of a surface by comparing the correspond to carbon dioxide. Negative values are
polarization of an incident beam with the reflected therefore changes of the air composition relative to
beam. The investigated depth is determined the time of calibration. Minor peaks at 2900 cm-1 are
evanescent field, which depends on the incident associated with CH2 or CH3 scissor vibration and
angle. might originate from organic solvents. Faint peaks at
3000 to 3700 cm-1 show OH stretching vibrations of
ATR-IR was done using a Bruker Hyperion 3000.
adsorbed or even embedded water.
This technique measures the absorption of the
evanescent field. Depending on the used materials In order to identify the chemical changes along
and illumination angle the investigated depth is in the the process chain the entire spectrum is fitted with
range of some hundreds of nanometers. individual peaks by means of a Gaussian profile
using Origin 2015 as shown in Figure 4 bottom. The
SNMS measurements were performed with a
area of the individual peak is then used as a measure
SPECS INA-X system. This technique removes the
for the presence of individual chemical compounds.
surface of the sample by argon ion sputtering. The
The results compared to the reference glass G101 is
ejected atoms from the surface are post-ionized and
shown in Figure 5. The most dominant modifications
detected in a mass analyzer. The investigated depth is
show the samples G103 (cleaning in diluted HF and
depending on the sputter time.
sodium carbonate rinse) and G105 (coating and
3. RESULTS decoating of chromium) with an enrichment of Al2O3
and AlSiO as well as a depletion of MgO,
3.1. MICROSCOPY respectively.
Investigation with optical microscopy shows
minor modifications along the process chain.
Differential interference contrast (DIC) microscopyFigure 4 Top: ATR-IR spectra with corresponding chemical Figure 6 Top: Comparison of FT-IR and ATR-IR spectra.
bonds. Bottom: Fit of the measured ATR-IR spectrum with Bottom: Fit of the measured FT-IR spectrum with multiple
multiple individual peaks. individual peaks.
The peaks correspond to ATR-IR peaks but in
particular Al2O3 and AlSiO are less intense.
Furthermore the differences of the individual glass
samples are less pronounced than in ATR-IR.
Therefore the variations of the areas of the
investigated samples are smaller. Nevertheless the
samples G103 and G105 show again an enrichment
of Al2O3 and AlSiO as well as a depletion of MgO,
respectively (c.f. Figure 7).
Figure 5: Intensity of the individual ATR-IR peaks along
the process chain.
3.4. IR-ELLIPSOMETRY
The signal of the IR-ellipsometry was used to
analyze the spectrum in a similar way to ATR-IR.
Figure 6 top shows both, ATR-IR and FT-IR spectra
of all samples. The spectrum was again decomposed
in single peaks corresponding to chemical
compounds (Figure 6 bottom).
Figure 7: Intensity of the individual IR ellipsometry peaks
along the process chain.3.5. ELLIPSOMETRY refractometer that was not able to detect any changes.
The thickest layer is present on sample G104 after HF
For the analysis with ellipsometry a model of the etching and ultra pure water (UPW) rinse.
system has to be fed in the data analysis system. We Simultaneously the absorption is very close to the
assumed a system of bulk glass (layer 0 in Figure 8) absorption of the reference. This indicates that the
with a gel layer (layer 1) and a top roughness of 1 nm etching process restored bulk properties but at the
(layer 2). same time, this fresh surface is vulnerable to attack
by water. The enhanced absorption of sample G106
(IBF and diluted HF dip) shows that IBF produces
residues on the surface that are not completely
Figure 8: Model of the glass surface used for the
removed by the diluted HF dip. The two samples
ellipsometry.
with the deviating IR-spectra G103 and G105 have
The optical properties of the bulk glass were elevated though not extreme values for n, k and d.
measured at a sample of freshly HF etched glass. The
obtained values for refractive index n and extinction 3.6. SNMS
coefficient k are kept constant for the consecutive The chemical depth profile of the reference
analysis. Using this model the optical properties n sample acquired by SNMS is shown in Figure 10.
and k and the thickness d of the gel layer are fitting Whereas most ions are depleted at the surface,
parameters for the analysis. The results of two sodium is strongly enriched.
different ellipsometric methods (mapping and
imaging ellipsometry) performed on two different
instruments are shown in Figure 9 combined with an
estimated mean squared error (MSE).
Figure 10: SNMS depth profile of the reference sample.
Both, silicon and oxygen show a depleted zone
within the first 10 to 20 nm under the surface but are
at bulk concentration at the very surface. In general
the depth of the gel layer is about 50 nm, which is
relatively few compared to other glasses (up to few
hundred nanometers). The silicon depletion zone is
considered a measure for strength of the glass
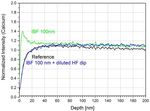
corrosion. Figure 11 shows the influence of
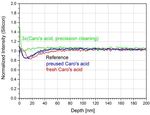
hydrofluoric acid, Caro’s acid and IBF on the silicon
depletion zone.
No major differences are visible for different HF
processes, which means that bulk properties with
some kind of “natural gel layer” are always present
Figure 9: Fitting results of mapping ellipsometry (top) and
after any kind of HF treatment. This is even true if the
imaging ellipsometry (bottom).
prior process was IBF. Whereas IBF alone alters
Both techniques reveal a qualitatively similar both, the silicon depletion layer (thinner) and the
result even though absolute numbers differ. The calcium concentration (c.f. Figure 12) at the surface
refractive index barely changes in both (higher), a consecutive dip in diluted hydrofluoric
measurements being consistent with the results of a acid restores typical properties. For Caro’s acid thebehavior is less obvious. A treatment with pre-used
Caro’s acid, which is less concentrated and of lower
temperature has no influence on silicon and calcium
concentration. When using fresh and hot (T > 100°C)
Caro’s acid the glass is affected approximately 10 nm
deeper. A triple treatment with fresh Caro’s acid with
intermediate ultrasonic and megasonic rinse in UPW
does not intensify this effect, instead inverses it. This
leads to nearly bulk properties nearly up to the very
top surface in terms of silicon concentration. At the
same time an enrichment of calcium is present at the
surface that might originate from calcium sulfate
formed by the reaction with the sulfuric acid of the
Caro’s mixture.
Figure 12: Comparative SNMS depth profiles for calcium
depending on different HF etching, IBF and Caro’s acid
processes.
4. SUMMARY
Different techniques are employed to investigate
the corrosive influence of different production
processes on the gel layer of borosilicate glass. In
general the degradation is small compared to other
glasses. Infrared methods show that hydrofluoric acid
with a consecutive soda rinse alters the glass surface
most significantly. Ellipsometry shows that etching
with hydrofluoric acid and UPW rinse leads to the
thickest gel layer. SNMS reveals that IBF and
repeated etching with Caro’s acid leave residues on
the surface but minimize the depth of the gel layer.
5. ACKNOWLEDGEMENTS
SNMS measurements were performed at
Clausthal University of Technology by Thomas
Peter. Ellipsometry and ATR-IR were performed at
at the Federal Institute for Materials Research and
Testing (BAM) with support of Uwe Beck, Andreas
Hertwig and Jennifa Baier. Sequential chemical
analysis was done at Aachen University by Klara
Sülz.
Figure 11: Comparative SNMS depth profiles for silicon
depending on different HF etching, IBF and Caro’s acid
processes.6. REFERENCES
[1] R. Conradt, "Chemical Durability of Oxide
Glasses in Aequeous Solutions: A Review,“
American Ceramic Society, pp. 728-735, 2007.
[2] E. Rädlein, "Glas und Witterung,“ in
Glastechnische Tagung der DGG, Amberg,
2009.
[3] P. S. Djambazov, "Theoretische und
experimentelle Untersuchungen der
Frühstadien bei der wässrigen Korrosion von
Silicatgläsern", Rheinisch-Westfälische
Technische Hochschule Aachen, 2014.
[4] V. P. Tolstoy, "Handbook of Infrared
Spectroscopy of Ultrathin Films", John Wiley &
Sons, 2003.You can also read