Metastatic Renal Cell Carcinoma to the Thyroid Gland
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
1869
Metastatic Renal Cell Carcinoma to the Thyroid Gland
A Clinicopathologic Study of 36 Cases
Clara S. Heffess, M.D.1 BACKGROUND. Clear cell tumors of the thyroid gland in general are uncommon.
Bruce M. Wenig, M.D.2 Metastatic renal cell carcinoma (RCC) to the thyroid gland is a rare occurrence but
Lester D. Thompson, M.D.1 must be considered in the differential diagnosis of a thyroid gland clear cell
neoplasm to prevent misclassification, potentially resulting in inappropriate clin-
1
Department of Endocrine and Otorhinolaryngic- ical management.
Head and Neck Pathology, Armed Forces Institute METHODS. Thirty-six cases of metastatic RCC to the thyroid were retrospectively
of Pathology, Washington, D.C. retrieved from the files of the Endocrine Registry of the Armed Forces Institute of
2
Department of Pathology, Beth Israel Medical Pathology.
Center, New York, New York. RESULTS. The tumors occurred in 22 women and 14 men, ages 53– 80 years (mean,
64.9 years). Symptoms were present for a mean of 13.0 months. The tumors
generally affected a single lobe of the thyroid gland as a solitary mass (n ⫽ 30; 83%),
measuring 1.0 –15.0 cm in diameter (mean, 3.8 cm). Histologically, the tumors were
composed of polygonal cells with clear cytoplasm, distinct cell membranes, and
small compact eccentric nuclei within a rich vascular network. Diastase-sensitive,
periodic acid–Schiff-positive material (n ⫽ 22; 61%) and/or Oil Red O-positive
material (n ⫽ 5; 14%) were noted. Thyroglobulin immunohistochemistry was
negative in the foci of metastatic RCC. Although the majority of the patients had
documented previous evidence of an RCC (n ⫽ 23; 64%) as remotely as 21.8 years
before the thyroid metastases (mean, 9.4 years), the metastatic tumor to the
thyroid gland was the initial manifestation of RCC in 13 patients. The majority of
patients (n ⫽ 23; 64%) died with disseminated disease (mean, 4.9 years), but 13
patients (36%) were alive or had died without evidence of disease (mean, 9.1 years).
CONCLUSIONS. In the presence of a clear cell tumor of the thyroid gland, the
diagnostic considerations must include metastatic RCC. The light microscopic
features may suggest this possibility and the diagnosis can be established by
supplemental histochemical and immunohistochemical studies. Surgical treat-
ment of the metastatic disease is suggested, as this may result in prolonged patient
survival. Cancer 2002;95:1869 –78.
Published 2002 by the American Cancer Society.*
The opinions or assertions contained herein are DOI 10.1002/cncr.10901
the private views of the authors and are not to be
construed as official or as reflecting the views of KEYWORDS: thyroid gland, renal cell carcinoma, metastatic disease, immunohisto-
the United States Department of Defense.
chemistry, surgery, prognosis.
Address for reprints: Clara S. Heffess, M.D., De-
partment of Endocrine and Otorhinolaryngic-Head
and Neck Pathology, Building 54, Room G066-9,
Armed Forces Institute of Pathology, 6825 16th
Street, NW, Washington, DC 20306-6000. Fax:
A metastatic neoplasm to the thyroid gland identified during life is
a distinctly uncommon cause of thyroid enlargement. Usually,
metastases are found at autopsy as part of widespread disease or they
(202) 782-3130; E-mail: heffess@afip.osd.mil directly invade the thyroid gland from a neoplasm from adjacent
organs such as the neck and/or mediastinum. Although secondary
Received March 26, 2002; revision received May
involvement of the thyroid gland by renal cell carcinoma (RCC) is
6, 2002; accepted May 23, 2002.
rare, it is still one of the more common neoplasms to metastasize to
*This is a US Government work and, as such, is in the thyroid gland (⬍ 0.1%).1– 8 When present, metastatic RCC can
the public domain in the United States of America. mimic primary thyroid gland neoplasms, potentially leading to diag-
Published 2002 by the American Cancer Society*1870 CANCER November 1, 2002 / Volume 95 / Number 9
nostic difficulties. This diagnostic dilemma is further All cases had hematoxylin and eosin-stained
complicated by several factors. These include its pres- slides for morphologic assessment of metastatic RCC.
ence as a solitary mass in the thyroid gland (most Periodic acid-Schiff (PAS) stain (with and without di-
often a solitary mass lesion of the thyroid gland is astase digestion), Mayer’s mucicarmine stain, and the
representative of a primary thyroid lesion) and its Oil Red O procedure were performed. Immunopheno-
occurrence in patients with no known history of an typic analysis was performed on a single block in 32
RCC (i.e., occult primary neoplasm) with the meta- cases with suitable material, according to the stan-
static deposits in the thyroid gland representing the dardized avidin-biotin method of Hsu et al.9 on 4-m
initial manifestation of their renal disease. thick, formalin-fixed, paraffin-embedded sections.
We undertook this study to underscore the impor- Commercially available immunohistochemical anti-
tance of the preoperative diagnosis of metastatic RCC bodies for cytokeratin (AE1/AE3/LP34: AE1/AE3,
to the thyroid gland. We attempt to define the histo- mouse monoclonal antibody [MoAb], 1:200, Dako,
logic criteria for the recognition of metastatic RCC, as Carpinteria, CA; LP34, mouse MoAb, 1:40, Boehringer
well as the utilization of adjunct histochemical and Mannheim Biochemicals, Indianapolis, IN) and thyro-
immunohistochemical stains in the diagnosis. In ad- globulin (mouse MoAb, 1:600, Dako) were used. Pre-
dition, we discuss the differential diagnosis of clear digestion was performed for 3 minutes with 0.05%
cell neoplasms of the thyroid gland and provide in- Protease VIII (Sigma, St. Louis, MO) in a 0.1 M phos-
sight into the appropriate clinical management of phate buffer, pH 7.8, at 37 °C. Appropriate standard
metastatic RCC to the thyroid gland. positive and negative (serum) controls were used
throughout. The lesional cells were graded as reactive
MATERIALS AND METHODS or nonreactive.
Thirty-six cases of metastatic RCC to the thyroid gland
were identified in the files of the Endocrine Registry at RESULTS
the Armed Forces Institute of Pathology from 1959 to Clinical Demographics and Presentation
1998. These cases were identified in a review of 37,158 There were 22 women and 14 men, ages 53– 80 years
(0.1%) benign and malignant thyroid neoplasms seen (mean, 64.9 years) at the time of clinically apparent
in consultation between 1959 and 1998. Secondary thyroid lesions (Table 1). The mean age at presenta-
clear cell tumors resulting from direct invasion from tion for women and men was 67.5 and 60.8 years,
malignant tumors of the contiguous organs were respectively, although this difference was not statisti-
omitted from consideration, as were cases of systemic cally significant. We separated the patients into two
disease (e.g., lymphoma). The cases included in this groups based on the type of presentation: RCC as the
study were obtained from civilian sources, including initial presentation (Group 1) and the thyroid tumor as
university medical centers and foreign contributors, the initial presentation (Group 2). There were 23 pa-
military hospitals, and Veterans Administration med- tients in Group 1 and 13 patients in Group 2. There
ical centers. was an overwhelming female predominance (11:2 fe-
Patient demographics, clinical symptoms at pre- male-to-male ratio) in Group 2, a finding similar to
sentation, and past medical and surgical history (spe- thyroid gland disorders in general. However, when
cifically, a history of RCC or renal surgery) were re- there was a known history of RCC, there was no dif-
viewed for all patients. In addition, we reviewed ference in the gender distribution (11 women and 12
radiographic, surgical pathology, and operative re- men). There was no appreciable difference in the
ports. We also obtained information from oncology mean age at presentation between Groups 1 and 2, the
data services by written questionnaires or direct com- location of the primary RCC, or the overall tumor size
munication with the physician(s) or patient. Follow- (Table 1).
up data, available for all patients, included informa- All patients experienced enlargement of the thy-
tion regarding tumor location, treatment modalities, roid gland (mass lesion), often with associated symp-
and current patient and disease status. With the ex- toms related to mass effect, such as hoarseness (com-
ception of one case in which there was only radiologic pression of the recurrent laryngeal nerve), difficulty
evidence of a neoplasm involving the kidney, all cases breathing, difficulty swallowing, and/or pain (n ⫽ 8).
included in this study have histologic confirmation of Thirty patients presented with a solitary nodule in the
RCC. This clinical investigation was conducted in ac- thyroid gland, three patients presented with multifo-
cordance and compliance with all statutes, directives, cal or bilateral disease, and three patients were noted
and guidelines of the Code of Federal Regulations, to have a mass of the thyroid during routine physical
Title 45, Part 46, and the Department of Defense Di- examination for other reasons. The symptoms were
rective 3216.2 relating to human subjects in research. present from 2 weeks to 10 years, with an overallMetastatic RCC to Thyroid Gland/Heffess et al. 1871
TABLE 1
Clinical Features of Metastatic Renal Cell Carcinomas to the Thyroid Gland
Renal cell carcinoma as Thyroid tumor as
All primary presentation primary presentation
All cases 36 23 13
Gender
Women 22 11 11
Men 14 12 2
Mean age at presentation (yrs)
All 64.9 65.3 64.2
Range 53–80 44–80 47–80
Women 67.5 69.2 65.9
Men 60.8 61.8 54.5
Length of symptoms (mos)
Range 0.5–120 0.5–52 0.5–120
Mean 13.0 8.3 21.0
Type of presentation
Solitary mass 30 19 11
Multifocal masses 3 3 0
Mass, not further specified 3 1 2
Anatomic location
Right 19 13 6
Left 11 6 5
Bilateral 3 3 0
Unknown 3 1 2
Tumor size (cm)
Range 1.0–15 1.0–15.0 1.5–4.5
Mean 3.8 4.1 3.2
average of 13.0 months. The patients with a known isolated clinical finding at the time of presentation,
RCC had a shorter mean duration of symptoms (8.3 even though other metastatic foci developed during
months) than patients without a known primary tu- the follow-up period. Follow-up information was
mor (21.0 months). This difference may be related to available for all patients (Table 2). All patients had
more frequent physical examinations and radio- clear cell RCC, without any primary chromophobe or
graphic studies for patients with a known primary papillary RCC. Thirteen patients were either alive (n
RCC tumor as part of their follow-up. Furthermore, ⫽ 8) or had died (n ⫽ 5) without evidence of disease
the overall longer mean duration of symptoms for (mean, 9.8 years), whereas 23 patients had died with
patients without a known primary tumor may be re- widely disseminated disease (mean, 4.9 years). These
lated to the generally nonspecific nature of the initial results yielded an overall raw 5-year survival rate of
presenting symptoms (e.g., slow enlargement of the 51.4% and a raw 10-year survival rate of 25.7%. Be-
thyroid gland), which often were managed symptom- cause thyroid metastases were already present, an
atically without a specific diagnostic evaluation. Even RCC disease-free survival rate is not applicable.
though a few patients had a long history of goiter (n To simplify the follow-up data, the patients were
⫽ 4), a change in size or symptoms prompted clinical grouped according to previous evidence of RCC as
attention. There was no clinical evidence of thyroid described above (Table 2). Furthermore, survival from
dysfunction (i.e., hypothyroidism or hyperthyroid- the date of the diagnosis of RCC also is presented
ism). (Table 2).
Clinical Management and Outcome Thyroid tumor as initial presentation
The thyroid masses were all managed by surgery, ir- In 13 patients (2 men and 11 women), the thyroid
respective of the treatment for the primary RCC (a few mass (enlargement) was the initial manifestation of
patients had been treated with radiation therapy for the disease. In these patients, the diagnosis of a clear
the primary RCC). The treatment included a lobec- cell RCC prompted a clinical and radiographic inves-
tomy in 21 patients and a total thyroidectomy in 15 tigation. A nephrectomy was performed between 4
patients. In all of our patients, the thyroid mass was an and 554 days after the diagnosis of a metastatic clear1872 CANCER November 1, 2002 / Volume 95 / Number 9
TABLE 2
Patient Outcome Based on Clinical Presentation (Follow-Up in Years)
Patient outcome A, NED D, NED D, D
All cases 8 5 23
Survival range from date of thyroid presentation 0.9–16.5 9.6–20.2 0.3–18.8
Survival mean from date of thyroid presentation 5.4 13.6 4.9
Years between primary and thyroid presentation (mean) 7.2 3.2 5.8
Survival from date of RCC diagnosis (mean) 13.7 16.8 10.8
Thyroid tumor as initial presentation 1 2 10
Range n/a 9.6–11.9 0.4–11.2
Mean 4.9 10.7 4.3
Survival from date of RCC diagnosis (mean) 4.8 10.4 4.0
RCC as initial presentation 7 3 13
Range 0.9–16.5 13.1–20.2 0.3–18.8
Mean 6.4 15.5 5.4
Years between primary and thyroid presentation (mean) 8.4 5.5 10.9
Survival from date of RCC diagnosis (mean) 14.9 21.0 16.5
A, NED: alive, no evidence of disease; D, NED: dead, no evidence of disease; D, D: dead with disseminated disease; RCC: renal cell carcinoma.
cell RCC to the thyroid in 11 of these patients. One Pathology
patient refused surgery and the primary RCC was dis- Macroscopic findings
covered in another patient at autopsy 1 year after The masses in the thyroid gland specimens were well
metastatic disease was diagnosed. One patient is alive circumscribed, encapsulated (contained within ad-
without evidence of disease at 4.9 years, two died enomatoid nodules that, by definition, do not have a
without evidence of disease (9.6 and 11.9 years, re- capsule), and varied from 1.0 cm to 15.0 cm in greatest
spectively), and 10 patients died with widely dissemi- diameter (mean, 3.8 cm). The masses in the thyroid
nated disease an average of 4.3 years after the thyroid gland in patients in whom the thyroid gland lesion
mass was diagnosed. These figures are slightly lower if was the initial presentation of the RCC were on aver-
the survival from the date of diagnosis of the RCC is age smaller (mean, 3.2 cm) than the masses in patients
calculated (Table 2). with a known primary RCC (mean, 4.1 cm). The num-
bers were too few to perform a meaningful statistical
analysis. The tumors were solitary masses in 30 pa-
RCC as initial presentation, but with subsequent thyroid tients and multifocal and/or bilateral in 3 patients.
enlargement This information was unavailable for three patients.
Of the 23 patients with a known RCC, 22 had a previ- The tumors involved the right lobe (n ⫽ 19) more
frequently than the left lobe (n ⫽ 11), but this differ-
ous nephrectomy for RCC from 2 to 21.9 years before
ence was not meaningful and did not relate to the side
the development of the thyroid gland metastases
of the primary RCC. It is noteworthy that none of the
(mean, 9.4 years). One patient had a clinically palpable
patients in whom the thyroid mass was the initial
mass, confirmed by radiographic images. Of these pa-
presentation of the RCC had multifocal and/or bilat-
tients, seven are alive without evidence of disease
eral disease.
(mean, 6.4 years after the thyroid presentation) and
On sectioning, the tumors appeared lobulated,
three had died without evidence of disease (mean, well demarcated, and sharply circumscribed or encap-
15.5 years after the thyroid presentation). The remain- sulated, from soft to partially necrotic and bright yel-
ing 13 patients died with widely disseminated disease low to reddish-tan (Fig. 1). The primary RCC affected
an average of 5.4 years after the thyroid mass was the left kidney in 20 patients, the right kidney in 8
diagnosed. The overall survival from the date of RCC patients. The particular side was unknown for eight-
diagnosis was much longer, as would be expected, patients. None of the patients had developed bilateral
with a mean survival of 14.9, 21.0, and 16.5 years, tumors.
respectively. At the time of death for the patients with
widely disseminated disease, the other metastatic foci Microscopic findings
included the vertebrae, lymph nodes, liver, lung, The metastatic foci were usually encapsulated (n ⫽ 19
pleura, brain, bones, and adrenal glands. cases). However, in many cases, the deposits of met-Metastatic RCC to Thyroid Gland/Heffess et al. 1873
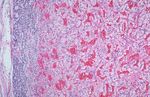
FIGURE 2. Low-power illustration of an encapsulated nodule of metastatic
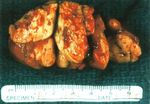
FIGURE 1. Metastatic renal cell carcinoma replacing the thyroid parenchyma. renal cell carcinoma in the thyroid gland.
The solid yellowish mass shows hemorrhage and degenerative changes.
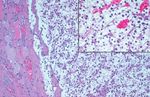
FIGURE 4. The characteristic vascular pattern with extravasated erythrocytes
FIGURE 3. The blending of the clear cells of a metastatic renal cell in a metastatic renal cell carcinoma.
carcinoma with cells of an adenomatoid nodule.
small, compact eccentric nuclei (Fig. 4). Nuclear ple-
astatic RCC were found within adenomatoid nodules omorphism was minimal to nonexistent. The cyto-
(n ⫽ 10), which are not by definition encapsulated or morphologic features of thyroid papillary carcinoma,
within follicular adenomas (n ⫽ 4; Figs. 2, 3). Although including nuclear enlargement, irregularities in nu-
well circumscribed in the majority, the cells were oc- clear size and shape, dispersed to optically clear ap-
casionally identified infiltrating the capsule and in- pearing nuclear chromatin, crowding or overlapping
vading small to medium vessels, making them virtu- nuclei, nuclear grooves, and nuclear inclusions, were
ally indistinguishable from follicular thyroid not identified in any of these foci. Although clear cell
carcinomas. The predominant histologic pattern was features were the dominant finding in all cases, foci of
characterized by the presence of small nests and cords cells with slightly eosinophilic cytoplasm were present
of neoplastic cells separated by a prominent vascular in eight cases. Hemorrhage (n ⫽ 6), a lymphoid infil-
stroma (Fig. 4). A primary thyroid follicular epithelial trate (n ⫽ 6), fibrosis (n ⫽ 2), microcysts (n ⫽ 2),
tumor was simulated by a “pseudofollicular” architec- microcalcifications (n ⫽ 2), and necrosis (n ⫽ 1) were
tural arrangement in nine cases in which cystic spaces also noted. The background thyroid parenchyma har-
were lined by clear cells and filled with erythrocytes. bored lymphocytic thyroiditis (n ⫽ 5) and microscopic
Furthermore, a tubular, angiomatoid, and a papillary papillary carcinoma (n ⫽ 2).
pattern as a minor component were present in two In the majority of the cases in which a capsule was
cases each, respectively. The neoplastic deposits were present, the neoplastic cells were identified infiltrating
composed of polygonal or elongated cells with clear the capsule with invasion of small to medium vessels.
cytoplasm, distinct cytoplasmic membranes, and This feature coupled with the histologic similarities to1874 CANCER November 1, 2002 / Volume 95 / Number 9
the neoplastic deposits, as the PAS reaction is also
used to accentuate colloid in the central luminal space
of thyroid follicular epithelial cells. No stainable mu-
cin was demonstrated in the neoplastic cells in any of
the cases evaluated. In a few cases with available ma-
terial (n ⫽ 5), numerous droplets of neutral fat (Oil
Red O stain) were demonstrated in the neoplastic
cells, not in the thyroid follicular cells.
All metastatic foci were nonreactive with thyro-
globulin. There were some difficulties in interpreta-
tion, especially in cases lying within or juxtaposed to
thyroid follicular epithelial cells. The thyroglobulin
staining in these cells was often very faint and non-
granular, representing diffusion rather than true im-
munoreactivity. As expected, diffuse and strong kera-
tin immunoreactivity was demonstrated in both the
metastatic and thyroid follicular cells. However, there
was a slight difference in the pattern of reactivity, with
an accentuation along the membranes in the meta-
static RCC cells, whereas a cytoplasmic reaction was
seen in the residual thyroid follicular epithelium.
DISCUSSION
Metastasis to the thyroid gland is an uncommon oc-
currence. However, autopsy results show that 1.9 –
22.4% of patients with generalized malignancies have
metastasis to the thyroid gland.10 The clinical and
histomorphologic features of most metastatic tumors
to the thyroid gland (reported in autopsy series) are
sufficiently distinctive to achieve separation without
any difficulty on the part of the clinician or patholo-
gist. According to one large autopsy series, malignant
melanoma (39%) and breast carcinoma (21%) account
for the largest number of tumors metastasizing to the
thyroid gland as part of widely disseminated disease
(excluding lymphoma and leukemia).5
Although the incidence of metastatic disease to
the thyroid gland in autopsy series is variable and is
compounded by the lack of systematic examination of
the thyroid gland during autopsy,11 antemortem man-
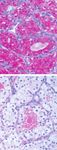
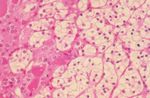
FIGURE 5. Glycogen-rich renal cell carcinoma cells. Periodic acid-Schiff
ifestations of metastatic disease are rare.8,12–14 The
(PAS; top) and PAS with diastase (bottom).
scarcity of antemortem evidence of metastatic disease
probably is related to the nonspecific nature of the
thyroid follicular epithelial tumors easily led to a di- symptoms. Clinical presentation of a mass lesion in
agnosis of thyroid follicular carcinoma. the thyroid gland that proves to be metastatic disease
is distinctly uncommon, with only a few cases of ma-
Special procedures lignant melanoma, breast carcinoma, and lung carci-
The metastatic foci contained variable amounts of noma identified in the files of the Armed Forces Insti-
diastase-sensitive, PAS-positive material, which was tute of Pathology or reported in the literature.12
indicative of glycogen in the cytoplasm of the clear However, when a thyroid mass presents as the clinical
cells (Fig. 5). The intensity varied from weak to strong, manifestation of metastatic disease, RCC seems to be
ranging from patchy (n ⫽ 15) to diffuse (n ⫽ 7) in the most frequent tumor type. In the absence of a
distribution. The PAS-positive material was only clinical history, the sudden enlargement of the thyroid
graded when identified within the cell cytoplasm in gland in an otherwise healthy patient makes the diag-Metastatic RCC to Thyroid Gland/Heffess et al. 1875
nosis of metastatic disease challenging. This is com- present in the remaining gland but not included in the
pounded further by histologic similarities between sections available for examination).
metastatic deposits and primary thyroid lesions. In contrast to this theory, others have suggested
RCCs are neoplasms of adulthood that are seen that vascular deterioration by itself would not be suf-
most frequently in the sixth decade of life with a male ficient to account for metastatic disease, that the fil-
predominance. However, because thyroid diseases in tering system of the lungs would probably remove
general are more frequent in women, the predomi- most tumor emboli, and that there was no difference
nance of female patients in this series is acceptable.15 in frequency of metastasis in altered thyroid glands
The average age of our patients at presentation of RCC versus normal thyroid glands.6,10,29 Whichever theory
(57.4 years, excluding the patients in whom the thy- may be correct, there is no explanation for the long
roid mass was the initial manifestation of the RCC) latent period between the identification of the primary
was similar to the findings reported in the literature. tumor (for the patients with a known renal primary)
RCC is known for its capacity to behave in an unpre- and the development of clinical thyroid gland metas-
dictable and unusual fashion. Metastatic foci from tases (mean, 9.4 years in this clinical study).12,14,30
RCC usually develop in the lower respiratory tract, Metastatic RCC may be the first manifestation of
skeletal system, lymph nodes, brain, liver, and skin, the disease, even masquerading as a primary thyroid
with other sites (such as the thyroid gland) described gland neoplasm. In the setting of a solitary thyroid
less frequently.16 –20 The recognition of metastatic foci mass many years after nephrectomy in a patient with
is important clinically because metastases usually sug- a long disease-free interval (arbitrarily defined as ⬎ 10
gest a poor prognosis, with the exception of a few years),16 the recognition that it may be an RCC may
tumors, such as RCC. It is peculiar that RCCs develop pose a diagnostic problem not only for the pathologist
but also for the clinician. The absence of symptoms
late and/or as a solitary metastasis. Although meta-
related to the urinary tract in many cases and some-
static foci are present in about 25% of RCCs at the time
times a failure to obtain a detailed clinical history may
of the diagnosis of the primary malignancy (synchro-
lead to an equivocal or incorrect diagnosis when the
nous),16,20 –24 metastatic disease can develop as part of
patient first presents with a thyroid gland mass.
the latency of the tumor with delayed development of
In none of the patients in our series was the RCC
metastases after many years of dormancy (metachro-
discovered simultaneously with the metastatic thyroid
nous). This is especially true if the primary carcinoma
tumor. Given the nature of our consultation service,
is clinically a low-stage malignancy.16,20,21,25 Moreover,
the history of RCC usually was disclosed within a few
a solitary metastasis from RCC occurs with an inci-
days of rendering the diagnosis. It is noteworthy that
dence rate of about 1– 4%,18,21,23,24,26 of which about
in 13 of our cases (36.1%), the metastatic focus was the
1% occurs in the thyroid gland. There are many tu-
initial presentation of the RCC. However, on average,
mors cells in the peripheral circulation, although only the metastatic focus developed 9.4 years after the ini-
a few form metastases. The presence of a solitary RCC tial nephrectomy (range, 2 months to 21.9 years). In
metastasis suggests the ability of the host to destroy fact, nine patients in this clinical series presented 10
the majority of the circulating neoplastic cells.25 years or more after the initial nephrectomy, reinforc-
The thyroid gland is one of the most vascularized ing the need for long-term clinical follow-up of pa-
organs in the body and one would expect it to be the tients with RCC.
site of metastatic disease. It has been suggested that Surgical treatment of patients with solitary thyroid
the thyroid gland, when altered by goiter, neoplasms, gland metastases is recommended because of the un-
or thyroiditis, is more vulnerable to metastatic growth usually good prognosis of our patients and of patients
due to metabolic changes with a decrease in oxygen reported in the literature when they were treated with
and iodine content.27 Beahrs et al.28 postulated that definitive surgical therapy. A number of factors may
the rich vascular supply inhibits the entrapment of be associated with a favorable prognosis after resec-
tumor emboli. However, when degeneration occurs in tion of the metastases, including 1) a long interval
neoplasms or in adenomatoid nodules, metastatic between the primary tumor resection and the devel-
neoplastic cells are readily deposited as a conse- opment of the metastatic focus (often in excess of 10
quence of the interrupted blood supply. In support of years as identified in this clinical series), 2) evidence of
this theory, adenomatoid nodules or adenomas were a solitary or isolated lesion in the thyroid gland with-
identified in this series in 10 patients, whereas chronic out evidence of widespread metastases, 3) spontane-
thyroiditis was noted in five patients. Therefore, 42% ous regression of metastatic lesions, 4) demonstration
of patients in this series had abnormal thyroid glands of extensive necrosis in the resected specimen, and 5)
(as a caveat, additional abnormalities may have been slow evolution or growth of the tumor and a lack of1876 CANCER November 1, 2002 / Volume 95 / Number 9
clinical symptoms.19,21,25,31 Moreover, spontaneous
remission or regression of cancer metastases is un-
common, but has been well described in RCC and
especially in RCC metastatic to the lung.19,25,32 None
of the patients in our series had a spontaneous regres-
sion, but all had surgery within a short time of docu-
menting metastatic disease rather than only being
followed clinically.
The mean survival rate reported in the literature is
variable and limited to case reports or small series
with a short follow-up period, making the analysis
unreliable for purposes of predicting survival. It has
been suggested that in the setting of solitary RCC
metastasis of any anatomic site, the 5-year survival
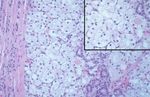
FIGURE 6. A clear cell follicular tumor of thyroid derivation. Inset: Colloid and
rate from the date of the nephrectomy ranges between
a lack of vascularity.
30% and 70%,16,20,21,23,26 which is much higher than
the approximately 5% 5-year survival rate when wide-
spread disease is present.16 In our series, the 5-year diagnosis with initiation of proper treatment, thereby
survival rate from the date of nephrectomy was 80% potentially increasing long-term patient survival. The
and the 10-year survival rate was 66%. In fact, the challenge of making the diagnosis of a metastatic clear
mean overall survival period from the date of the cell RCC in the thyroid gland on intraoperative consul-
nephrectomy was 12.3 years (range, 0.2–31.5 years), tation (i.e., frozen section) is difficult due to fixation
with a 6.4-year mean overall survival from the date of artifact that makes the cytoplasm appear more eosino-
the thyroid metastasis (range, 0.3–20.2 years). There- philic than clear in appearance. However, architectural
fore, there is an overall excellent prognosis for RCC features, such as the presence of fibrovascular cores and
patients with solitary metastasis (disease stage was not erythrocytes within the pseudofollicles, may assist in
reported for the original material), further highlighting making an accurate distinction between a primary tu-
the necessity for surgical resection of solitary meta- mor and metastatic RCC. However, in practicality, this is
static foci to the thyroid gland to assure the possibility a very challenging differential diagnosis at intraoperative
of a favorable clinical outcome.21 consultation. RCCs and thyroid follicular epithelial neo-
The preoperative distinction between a primary ver- plasms with clear cells may have a variety of histologic
sus a secondary thyroid neoplasm is almost impossi- patterns that make a distinction on morphologic
ble.31,33 Radiographic differences cannot be used to dis- grounds alone difficult. RCCs may have pseudofollicles
criminate between primary or metastatic tumor as both filled with blood. The cells have clear cytoplasm, distinct
of these lesions will appear as “cold” nodules on radio- boundaries, and small, compact, dark nuclei. Thyroid
iodine uptake studies or as an “inhomogeneous, hypo- follicular epithelial tumors with clear cells are rare and
echoic” mass on ultrasound. Therefore, radiographic im- include follicular adenoma, follicular carcinoma, and
aging is not helpful in differentiating a primary thyroid papillary carcinoma. The clear cell component within a
neoplasm from a metastasis to the thyroid gland. The primary thyroid follicular neoplasm may be the domi-
true metastatic nature of the tumor is recognized only nant cell type or may represent a minor component of
after tumor sampling with pathologic assessment as the entire neoplastic proliferation (Fig. 6). The presence
there is no clinical pattern associated with metastatic of clear cells in any thyroid follicular neoplasm does not
lesions that differentiate it from a primary thyroid can- alter the overall prognosis of that particular tumor type.
cer. Therefore, all patients for whom there is clinical or The presence of large amounts of glycogen (diastase
radiographic evidence of a mass (particularly in solitary sensitive, PAS positive), large amounts of lipids,15 and
masses) in the thyroid gland require a fine-needle aspi- the absence of mucin favor the diagnosis of RCC. In
ration biopsy or a core-needle biopsy of the mass, irre- contrast, thyroid tumors with clear cells do not have
spective of whether the patient has a known history of a intracytoplasmic glycogen, although moderate amounts
previous malignancy (RCC or another tumor type) or no are seen occasionally. It is important to emphasize that
known primary malignancy elsewhere.1,5,13,29 –34 If the colloid droplets always stain with PAS, but are diastase
patient is known to have a previous malignancy of renal resistant. Variable amounts of neutral fat (Oil Red O
origin, then the suspicious radiographic lesion should be technique) are seen in lipid-rich thyroid primary tumors,
surgically excised rather than have the patient undergo a which are virtually indistinguishable from RCCs. Studies
biopsy of the lesion. This would allow for an accurate on lipid-rich thyroid follicular lesions have shown thatMetastatic RCC to Thyroid Gland/Heffess et al. 1877
scattered neutral lipid droplets occur intracellularly and noma metastasizing to the thyroid gland. Scand J Urol
their accumulation represents a degenerative phenom- Nephrol. 1999;33:202–204.
8. Shimaoka K, Sokal JF, Pickren JW. Metastatic neoplasms in
enon that increases with age. Therefore, when a lipid-
the thyroid gland. Pathological and clinical findings. Cancer.
rich thyroid primary neoplasm is suspected, additional 1962;15:557–565.
special studies are needed to assist in rendering the 9. Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase
diagnosis.35,36 The majority of RCCs are keratin and ep- complex (ABC) in immunoperoxidase techniques: a com-
ithelial membrane antigen immunoreactive, whereas parison between ABC and unlabeled antibody (PAP) proce-
they are nonreactive with thyroglobulin and thyroid dures. J Histochem Cytochem. 1981;29:577–580.
10. Willis RA. Metastatic tumors in the thyroid gland. Am J
transcription factor-1 (TTF-1). Primary thyroid follicular
Pathol. 1931;7:187–208.
epithelial tumors are also immunoreactive with keratin, 11. Silverberg SG, Vidone RA. Metastatic tumors in the thyroid.
but stain strongly and diffusely with thyroglobulin and Pac Med Surg. 1966;74:175–180.
TTF-1. These antigenic profiles allow for accurate and 12. Wichulis AR, Beahrs OH, Woolner LB. Metastasis of carci-
reliable separation of RCCs from primary thyroid follic- noma to the thyroid gland. Ann Surg. 1964;160:169 –177.
ular epithelial neoplasms.35– 41 13. McCabe DP, Farrar WB, Peltkov TM, Finkelmeier W,
O’dwyer P, James A. Clinical and pathological correlations in
In summary, the identification of a clear cell tu-
disease metastatic to the thyroid gland. Am J Surg. 1985;150:
mor of the thyroid gland must be evaluated to exclude 519 –523.
the possibility of metastatic RCC. This is especially 14. Elliot RHE, Frantz V. Metastatic carcinoma masquerading as
true when it is found in a patient with a previous primary thyroid cancer: a report of authors’ of 14 cases. Ann
history of RCC, irrespective of the temporal sequence Surg. 1960;151:551–561.
from the previous RCC. Although the clinical manifes- 15. Green LK, Ro JY, Mackay BAA, Luna MA. Renal cell carci-
noma metastatic to the thyroid. Cancer. 1989;63:1810 –1815.
tations and radiographic findings are often nonspe-
16. McNichols DW, Segura JW, DeWeerd JH. Renal cell carci-
cific, the architectural, cytologic, histologic, histo- noma: long-term survival and late recurrence. J Urol. 1981;
chemical, and immunohistochemical features are 126:17–23.
sufficiently distinctive to allow differentiation of a pri- 17. Riches MS, Griffiths IH, Thackray AC. New growths of the
mary thyroid follicular epithelial neoplasm from RCC. kidney and ureter. Br J Urol. 1951;23:297–356.
It is important to distinguish between a primary thy- 18. Saitoh H. Distant metastasis of renal adenocarcinoma. Can-
cer. 1981;48:1487–1491.
roid follicular epithelial neoplasm and metastatic RCC
19. Dekernion JB, Ramming KP, Smith RB. The natural history
to correctly manage the patient, especially in the pres- of metastatic renal cell carcinoma: a computer analysis.
ence of an occult RCC. The course of RCC is unpre- J Urol. 1978;120:148 –152.
dictable and the thyroid lesion may represent the only 20. Ritchie AW, Chisholm GD. The natural history of renal car-
manifestation of the disease. Surgical treatment of the cinoma. Semin Oncol. 1983;10:390 – 400.
solitary metastatic deposit is recommended as the 21. Kierney PC, van Heerden JA, Segura JW, Weaver LA. Sur-
geon’s role in the management of solitary renal cell carci-
patient may enjoy a prolonged survival. Surgery is
noma metastases occurring subsequent to initial curative
even more beneficial if the RCC was known before the nephrectomy: an institutional review. Ann Surg Oncol. 1994;
metastasis, as determined in this clinical series. 1:345–352.
22. Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbet-
ter WF. Diagnosis and management of renal cell carcinoma.
REFERENCES A clinical and pathologic study of 309 cases. Cancer. 1971;
1. Czech JM, Lichtor TR, Carney JA, van Heerden JA. Neo-
28:1165–1177.
plasms metastatic to the thyroid gland. Surg Gynecol Obstet.
23. Tolia BM, Whitmore WF, Jr. Solitary metastasis from renal
1982;155:503–505.
cell carcinoma. J Urol. 1975;114:836 – 838.
2. de Lima MA, Hasashy EM, de Lander Schmitt FC. Metastase de
24. Weerdenburg JP, Jurgens PJ. Late metastases of a hyper-
carcinoma de rim para tireoide dezessete anos apos a ressecao
do tumor primario [Metastasis of kidney carcinoma to thyroid nephroma to the thyroid and the pancreas. Diagn Imaging
gland 17 years after resection of the primary tumor]. Rev Hosp Clin Med. 1984;53:269 –272.
Clin Fac Med Sao Paulo. 1996;51:138–140. 25. Rubin P. Comment: are metastases curable? JAMA. 1968;204:
3. Gault EW, Leung THW, Thomas DP. Clear cell renal carci- 612– 613.
noma masquerading as a thyroid enlargement. J Pathol. 26. O’dea MJ, Zincke H, Utz DC, Bernatz PE. The treatment of
1974;113:21–25. renal cell carcinoma with solitary metastasis. J Urol. 1978;
4. Ivy HK. Cancer metastatic to the thyroid: a diagnostic prob- 120:540 –542.
lem. Mayo Clin Proc. 1984;59:856 – 859. 27. Linton RR, Barney JD, Moorman HD, Lerman J. Metastatic
5. Lasser A, Rothman JG, Calamia VJ. Renal-cell carcinoma hypernephroma of the thyroid gland. Surg Gynecol Obstet.
metastatic to the thyroid. Aspiration cytology and histologic 1946;43:493– 498.
findings. Acta Cytol. 1985;29:856 – 858. 28. Beahrs OH, Ginsberg RL, Miller GE. Metastatic hyper-
6. Lehur PA, Cote RA, Poisson J, Boctor M, Elhilali M, Kandalaft nephroma of the thyroid gland. Proc Mayo Clin. 1953;28:
N. Thyroid metastasis of clear-cell renal carcinoma. Can 205–209.
Med Assoc J. 1983;128:154 –156. 29. Mortensen JD, Woolner LB, Bennett WA. Secondary malig-
7. Palazzo FF, Bradpiece HA, Morgan MWE. Renal cell carci- nant tumors of the thyroid gland. Cancer. 1956;9:306 –309.1878 CANCER November 1, 2002 / Volume 95 / Number 9
30. Shima H, Mori H, Takahashi M, Nakamura S, Miura K, Tarao the thyroid, oxyphilic cell type, “clear cell” variant. A light
M. A case of renal cell carcinoma solitarily metastasized to and electron-microscopic study. Am J Surg Pathol. 1980;4:
thyroid 20 years after the resection of primary tumor. Pathol 501–509.
Res Pract. 1985;179:666 – 670. 37. Civantos F, Albores-Saavedra J, Nadji M, Morales AR. Clear
31. Flanigan RC. Role of surgery in patients with metastatic cell variant of thyroid carcinoma. Am J Surg Pathol. 1984;8:
renal cell carcinoma. Semin Urol Oncol. 1996;14:227–229. 187–192.
32. Garfield DH, Kennedy BJ. Regression of metastatic renal cell 38. Harach HR, Franssila KO. Thyroglobulin inmunostaining in
carcinoma following nephrectomy. Cancer. 1972;30:190–196. follicular thyroid carcinoma: relationship to the degree of
33. Ericsson M, Biorklund A, Cederquist E, Ingemansson S, Ak- differentiation and cell type. Histopathology. 1988;13:43–54.
erman M. Surgical treatment of metastatic disease in the 39. Schroder S, Bocher W. Lipomatous lesions of the thyroid
thyroid gland. J Surg Oncol. 1981;17:15–23. gland: a review. Appl Pathol. 1985;3:140 –149.
34. Links JA, Franzen S. Aspiration cytology of metastatic hy- 40. Schroder S, Bocher W. Clear-cell carcinomas of thyroid
pernephroma. Acta Cytol. 1984;28:250 –260. gland: a clinicopathological study of 13 cases. Histopathol-
35. Carcangiu ML, Sibley RK, Rosai J. Clear cell change in pri- ogy. 1986;10:75– 89.
mary thyroid tumors. A study of 38 cases. Am J Surg Pathol. 41. Shimizu KNM, Kitamura Y, Chin K, et al. Clinicopathological
1985;9:705–722. study of clear-cell tumors of the thyroid: an evaluation of 22
36. Dickersin RG, Vickery AL, Smith SB. Papillary carcinoma of cases. Jpn J Surg. 1995;25:1015–1022.You can also read