E S C Redefining susceptibility categories - and introducing the "area of technical uncertainty." - ESCMID
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
a r
ib r
e L
Redefining susceptibility categories
I D or
and introducing the ”area of technical uncertainty.”
CM t h
u
Gunnar Kahlmeter
E S a
gunnar.kahlmeter@eucast.org
Clinical microbiology
Växjö, Swedena r
In 2019 EUCAST decided …..
ib r
e L
• To change the definitions of S, I and R.
I D or
• To retain the letters S, I och R.
M
• To emphasize the relationship between the concentration of
t h
the antimicrobial agent at the site of the infection AND the
C u
breakpoints for categorisation (S, I and R).
S a
• To task clinical laboratories with the responsibility for
E
uncertain laboratory results, irrespective of origin.
Gunnar Kahlmeter, EUCAST 2019a r
ib
All breakpoints are ”exposure dependant”. r
e L
• Unless the microorganism is sufficiently exposed to the
antimicrobial at the site of infection, there will be no
I D or
inhibitory or killing effect.
M
• The degree of exposure is determined by the agent and its
C t h
pharmacokinetics in the patient, the individual dose, the
u
frequency of dosing and the mode of administration.
E S a
• For some agents there is only one dose and one mode of
administration – for others there are many options.a r
Susceptibility categories S, I and R (2002 – 2018)
ib r
e L
Definitions of S and R were straightforward:
D or
S = a micro-organism is defined as susceptible by a level of antimicrobial activity associated
I
with a high likelihood of therapeutic success.
I = a microorganism is defined as intermediate by a level of antimicrobial agent activity
M
associated with uncertain therapeutic effect. It implies that an infection due to the isolate
h
may be appropriately treated in body sites where the drugs are physiologically
C t
concentrated or when a high dosage of drug can be used; it also indicates a buffer zone
u
that should prevent small, uncontrolled, technical factors from causing major
S
discrepancies in interpretations.
E a
R = a micro-organism is defined as resistant by a level of antimicrobial activity associated
with a high likelihood of therapeutic failure.
Gunnar Kahlmeter, EUCAST 2019a r
ib r
• Uncertain therapeutic effect – responsibility of EMA, EUCAST and the
company
e L
– The breakpoint committee is responsible for breakpoints and guidance.
– The validity of indications, breakpoints and methods.
I D or
• Uncertain result – responsibility of the laboratory
M
– The laboratory is responsible for AST results.
C t h
– Methodological and/or interpretative uncertainty (failing method)
u
S
• Concentration at the site of infection – responsibility of the clinician
E a
– Dosing/administration: dose, frequency, mode (oral, iv, infusion).
Gunnar Kahlmeter, EUCAST 2019a r
It was impossible to know which of 3 – 4 meanings
were valid.
ib r
e L
• So INTERMEDIATE was avoided!
• We lost respect for ”INTERMEDIATE”
I D or
• We often lumped it with ”R”:
CM t h
– Some converted I to R in the report
– Others lumped ”I” and ”R” together as ”Non-susceptible” in
S u
surveillance.
a
E Gunnar Kahlmeter, EUCAST 2019a r
VME (false susceptible result)
ME (false resistant result)
ib r
e L
The wider the I-category, the less likely VMEs and MEs occur.
However, a wide I-category creates results with uncertain interpretation!
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
SIR – previous definitions
ib r
Susceptible
e L Intermediate
Uncertain effect.
Buffer zone for technical variation.
Resistant
D or
For a high dose.
I
Where concentrated for pharmacokinetic reasons.
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
New definitions of S, I and R were needed…
ib r
e L
S - Susceptible, standard dosing regimen: A microorganism is categorised as
Susceptible, standard dosing regimen, when there is a high likelihood of therapeutic
success using a standard dosing regimen of the agent.
I D or
I – Susceptible, increased exposure: A microorganism is categorised as Susceptible,
M
Increased exposure* when there is a high likelihood of therapeutic success because
h
exposure to the agent is increased by adjusting the dosing regimen or by its
C t
concentration at the site of infection.
S a u
R - Resistant: A microorganism is categorised as Resistant when there is a high
E
likelihood of therapeutic failure even when there is increased exposure.
* Exposure is a function of how the mode of administration, dose, dosing interval, infusion time, as well as distribution and excretion of the antimicrobial
agent will influence the infecting organism at the site of infection.
Gunnar Kahlmeter, EUCAST 2019a r
SIR – new definitions 2019
ib r
e L
Susceptible Resistant
I D or
Normal Increased
M
exposure exposure
C u t h
E S a Gunnar Kahlmeter, EUCAST 2019a r
SIR – new definitions 2019
ib r
e L
Susceptible Resistant
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
Carbapenems
Doripenem
Ertapenem
Standard dose
1 g x 1 iv over 30 minutes
High dose
None
ib r Special situations
L
Imipenem 0.5 g x 4 iv over 30 minutes 1 g x 4 iv over 30 minutes Pseudomonas spp.: High dose only
Acinetobacter spp.: High dose only
Meropenem 1 g x 3 iv over 30 minutes 2 g x 3 iv over 30 minutes Meningitis: 2 g x 3 iv over 30
e
EUCAST – dosing and minutes
Monobactams administration of antibiotics and
Standard dose High dose Special situations
D or
Aztreonam 1 g x 3 iv 2 g x 4 iv Pseudomonas spp.: High dose only
I
the relationship to breakpoints.
Fluoroquinolones Standard dose High dose Special situations
M
Ciprofloxacin 0.5 g x 2 oral or 0.4 g x 2 iv 0.75 g x 2 oral or 0.4 g x 3 iv Pseudomonas spp.: High dose only
h
Acinetobacter spp.: High dose only
t
Staphylococcus spp.: High dose
C
only
Gonorrhoea: 0.5 g oral as a single
u
dose
S
Levofloxacin 0.5 g x 1 oral or 0.5 g x 1 iv 0.5 g x 2 oral or 0.5 g x 2 iv Pseudomonas spp.: High dose only
a
Acinetobacter spp.: High dose only.
E
S. pneumoniae: High dose only
Moxifloxacin 0.4 g x 1 oral or 0.4 g x 1 iv None
Norfloxacin 0.4 g x 2 oral None
Ofloxacin 0.2 g x 2 oral or 0.2 g x 2 iv 0.4 g x 2 oral or 0.4 g x 2 iv Staphylococcus spp.: High dose
only
Gunnar Kahlmeter, EUCAST 2019a r
Terminology in speech and writing
ib r
•
•
e L
Report the bacterium S, I or R (laboratory report)
The isolate is S, I or R to the agent in question.
D or
• The isolate belongs to the S, I or R category.
•
I
The isolate is categorised as susceptible at normal dosing, susceptible at
M
increased exposure and resistant.
h
• The isolate can be called susceptible or resistant (but not intermediate)
C t
• Isolates which test S or I are called susceptible.
S a u
• ”Non-susceptible”, which was used to describe isolates which were I or R,
E
now only describes resistant isolates.
Gunnar Kahlmeter, EUCAST 2019a r
Consequences of the new S, I and R……
ib r
L
in the clinic.
e
• Agents with an “I” in the laboratory report must be considered a
therapeutic alternative – some will never be “S”.
I D or
• The need for increased exposure must be considered.
• A number of wild type populations, hitherto categorised as “Susceptible”
M h
(but with a note in tables that high dose is required) will be re-categorised
C t
“I” (Susceptible, increased exposure).
u
– Some of these are already implemented in v 9.0, 2019.
S a
• Examples Acinetobacter vs. fluoroquinolones; Proteii vs. imipenem.
E
– Others are under development and will appear during 2019 for consultation.
Gunnar Kahlmeter, EUCAST 2019a r
Consequences of the new S, I and R…..
ib r
L
in the laboratory.
e
”I” as a methodological buffer is gone.
I D or
The laboratory can no longer “hide” behind an “intermediate” – you
need to get it right and QC your results, irrespective of what method you
M
are on.
C t h
It also places major responsibility on manufacturers of devices, panels,
u
S
disks, agar, to make sure their products and material are up to speed and
a
can be quality controlled in daily microbiology.
Ea r
ib r
e L
I D or
CM
Tuesday 21.30
t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
A logical progression…
ib r
e L
• In 2019 year´s table, there are a number of S-breakpoints
qualified with the superscript ”HE” (for high exposure).
I D or
• During 2019 we will consult the world at large on the following
M h
proposal…..the logical progression of the new definitions.
C t
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
L
In several instances there will only be…..
e
D Ror
I Iand
CM t h
u
.…for some organisms and agents there will never
E S a
be ”S”.
Gunnar Kahlmeter, EUCAST 2019a r
Species Agent Current
breakpoint
ib r
Proposed new
breakpoint
From ”S” to
L
Enterobacterales Cefuroxime IV 8/8HE 0.001/8 I
Species
e Agent Current Proposed new From ”S” to
D or
breakpoint breakpoint
M I
P. aeruginosa
Piperacillin-tazo
Ticarcillin
16/16HE
16/16HE
0.001/8
0.001/8
I
I
h
Preliminary, decision and
Cefepime consultation
8/8HE 0.001/8 pending
I
S C u t
Ceftazidime
Aztreonam
8/8HE
16/16HE
0.001/8
0.001/16
I
I
a
Ciprofloxacin 0.5/0.5HE 0.001/0.5 I
E
Levofloxacin 1/1HE 0.001/1 I
Imipenem 4/4HE 0.001/4 I
Gunnar Kahlmeter, EUCAST 2019a r
Species Agent Current
ib r
Proposed new From ”S” to
breakpoint breakpoint
e
S.maltophilia
L
Trimethoprim-
sulfamethoxazole
4/4 0.001/2 I
D or
Staphylococcus Ciprofloxacin 1/1HE 0.001/1 I
I
Levofloxacin 1/1HE 0.001/1 I
M
Preliminary,Ofloxacin
decision and1/1consultation
HE 0.001/1 pending
I
t h
Strc. A,C,G Levofloxacin 2/2 0.001/2 I
C
S. pneumoniae Levofloxacin 2/2 0.001/2 I
S a u
H.influenzae Amoxicillin (oral) 2/2 0.001/2 I
E Amoxi-clav (oral) 2/2
Gunnar Kahlmeter, EUCAST 2019
0.001/2a r
ib r
ATUe L
I D or
CM t h
The Area of Technical Uncertainty
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
i b r
Most testing AST is unproblemtic
e L
…if your method is robust
I D or
…if your device is QC:ed
M
…if you trust and QC your gradient test
C t h
…if your disks are high quality
u
S
…if your MH medium is dependable
E a
…if the agent and the species cooperate
Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
Variability in MIC and disk testing
ib r
e L
Trained professionals with good quality material
D or
can 90-95% of the time attain:
M I
h
• a target MIC value +/- 1 dilution
S C u t
• a target zone diameter +/- 2 mm
E a Gunnar Kahlmeter, EUCAST 2019a r
ib r
Repeat MIC testing using broth quality controlled brothmicro trays .
L
The best you can achieve!
e
D or
Almost all results on target
M I +/- 1 MIC dilution.
C u t h
E S a Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
Ea r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
BUT, sometimes there is a need for a “warning”
ib r
e L
• Variation in the method which is difficult to control.
D or
• Variation in the interpretation which is difficult to control
M I
– Breakpoint splits wild type (mostly avoided by EUCAST)
– Breakpoint splits an important resistant population (ceftaroline in
C
MRSA)
u t h
E S a Gunnar Kahlmeter, EUCAST 2019a r
Area of Technical Uncertainty (ATU)
ib r
e L
• ATU is not a fourth susceptibility category – it is to warn laboratory
staff about an area where interpretation is difficult.
I D or
• The ATU is not to compensate for poor methodological skills – on
the contrary, AST today require more skills than ever before.
CM t h
• ATU is defined by a single MIC-value or a short range of zone
S u
diameter values.
E a
• How the ATU is dealt with depends on the situation (the sample,
the agent, the infecting organism).
Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
E. coli and K.pneumoniae from the Merino trial
ib
Courtesy Andrew Henderson, Brisbane, Australia r
L
Courtesy Andrew Henderson, Brisbane, Australia
e
I D or
CM t h
S a u
Ea r
Current ATUs (2019)
ib r
e
• Enterobacterales
L amoxicillin-clavulanic acid (systemic)
piperacillin-tazobactam
I D or
ciprofloxacin
• Ps. aeruginosa piperacillin-tazobactam
•
CM
S. aureus
t h
ceftazidime-avibactam
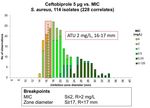
ceftaroline, ceftobiprole
•
S a u
S. epidermidis MRSE cefoxitin screen test
E
• H. influenzae with PBP3-mutations (betalactams)
Gunnar Kahlmeter, EUCAST 2019a r
EUCAST breakpoint table v.9.0 (2019) with columns for ATU
ib r
Penicillins1
e L MIC breakpoint
S≤
(mg/L)
R> ATU
Disk
content
(µg)
Zone diameter
breakpoint (mm)
S≥ R< ATU
Notes
Numbered notes relate to general comments and/or MIC breakpoints.
Lettered notes relate to the disk diffusion method.
D or
Benzylpenicillin - - - - 1/A. Wild type Enterobacterales are categorised as susceptible to aminopenicillins.
81 14A,B 14B Some countries prefer to categorise w ild type isolates of E. coli and P. mirabilis as "Susceptible, increased
I
Am picillin 8 10
Am picillin-sulbactam 81,2 82 10-10 14A,B 14B exposure". When this is the case, use the MIC breakpoint S ≤ 0.5 mg/L and the corresponding zone diameter
1 C breakpoint S ≥ 50 mm.
Am oxicillin 8 8 - Note NoteC
2. For susceptibility testing purposes, the concentration of sulbactam is fixed at 4 mg/L.
Am oxicillin-clavulanic acid 81,3 83 20-10 19A,B 19B 19-20
3. For susceptibility testing purposes, the concentration of clavulanic acid is fixed at 2 mg/L.
Am oxicillin-clavulanic acid 321,3 323 20-10 16A,B 16B 4. For susceptibility testing purposes, the concentration of tazobactam is fixed at 4 mg/L.
M
(uncom plicated UTI only) 5. Breakpoints still under consideration.
Piperacillin 8 16 30 20 17 6. Agar dilution is the reference method for mecillinam MIC determination.
h
Piperacillin-tazobactam 84 164 16 30-6 20 17 17-19
t
Ticarcillin 8 16 75 23 20 B. Ignore grow th that may appear as a thin inner zone on some batches of Mueller-Hinton agars.
C
Ticarcillin-clavulanic acid 83 163 75-10 23 20 C. Susceptibility inferred from ampicillin.
D. Ignore isolated colonies w ithin the inhibition zone for E. coli.
Tem ocillin Note5 Note5 Note5 Note5
u
Phenoxym ethylpenicillin - - - -
S a
Oxacillin - - - -
Cloxacillin - - - -
E
Dicloxacillin - - - -
Flucloxacillin - - - -
Mecillinam (uncom plicated UTI only) 86 86 10 15D 15D
E. coli, Klebsiella spp. (except K.
aerogenes ), Raoultella spp. and
P. mirabilis
Gunnar Kahlmeter, EUCAST 2019a r
ATU –alternative actions
ib r
e L
• Repeat the test –if test failed technically.
D or
• Confirm using an alternative test (MIC, PCR, PBP-
I
agglutination…).
M h
• Report the result with comment – “uncertain result”.
C u t
• Down-grade interpretation: S to I, I to R.
S
E a
• Discuss and explain to clinical colleagues.
Gunnar Kahlmeter, EUCAST 2019a r
Try hard…
ib r
e L
• IF only few alternative antibiotics for therapy.
• IF in a positive blood culture (or other serious infection).
I D or
• IF it can be easily solved.
CM t h
S u
• BUT if there are many alternatives, THEN report blank
a
E
and add a comment.
Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
Thank you!
I D or
M
gunnar.kahlmeter@eucast.org
C u t h
E S a Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
3. RAST – Rapid AST directly from
I D or
blood culture bottles
M t h
Emma Jonasson, Erika Matuschek, Martin
C u
Sundqvist, Anna Åkerlund, Gunnar Kahlmeter
E S a
On behalf of EUCAST
Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
Snabb resistensbestämning direkt från
blododlingsflaskor
ib r
•
•
e L
Art-ID på 60 min (87%)
Resistensbestämning inom 4, 6 eller 8 timmar.
D or
• 100 – 150 uL direkt från blododlingsflaskan till MH/MHF-medier
I
• Omedelbar inkubation och avläsning efter 4, 6 och 8 timmar.
•
M
Rapportering: endast S och R
h
• Lämna blankt om i ATU eller zonen ej tydligt läsbar. - inkubera
C t
omedelbart igen och läs efter 6 och 8 timmar.
u
• Om osäkert/inget resultat efter 8 timmar – utför standardiserad 16 –
E S a
20 h resistensbestämning.
• Flödet på laboratoriet avgör den diagnostiska gången och
möjligheterna.
Gunnar Kahlmeter, EUCAST 2019a r
Species
ib r
e L
The method is currently validated for the following species.
– Escherichia coli
D or
– Klebsiella pneumoniae
I
– Pseudomonas aeruginosa (6, 8h)
– Staphylococcus aureus
CM t h
– Streptococcus pneumoniae
– Enterococcus faecalis and Enterococcus faecium
S a u
• Acinetobacter, S.epidermidis ….
E
• More antibiotics…
Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
”Blank results”
ib r
e L
Laboratoriet bör överväga att inkludera en kommentar som
förklarar varför visa resultat lämnats blanka I
I D or
resistensbeskedet:
CM t h
”Resistensbestämning med tidig avläsning av resultat (4, 6
eller 8 timmar) förutsätter att endast pålitliga resultat
S u
rapporteras. Ett blankt resultat i en rapport kan följas av ett
a
E
pålitligt resultat i en senare avläsning.”
Gunnar Kahlmeter, EUCAST 2019a r
The EUCAST RAST clinical breakpoint are based on data
from three studies.
ib r
1.
e L
Spiked bottles with selected difficult isolates, performed at
EDL. Isolates have been tested with the RAST method on MH-
agar from Oxoid and BD/BBL.
D or
Reference method was BMD.
2.
M I
Clinical trial northern Europe, clinical isolates from 40
laboratories. Locally used MH-agars and antimicrobial discs.
Reference method is EUCAST disk diffusion 16-20 h (validated
h
against BMD).
3.
S C u t
Clinical trial southern Europe, clinical isolates from 15
laboratories. Locally used MH-agar and antimicrobial discs.
a
Reference method is EUCAST disk diffusion 16-20 h.
E Gunnar Kahlmeter, EUCAST 2019a r
Blood culture bottles, media and disks used
ib r
e
-Bactec
L
Blood culture bottles
-BactAlert (old and new)
Disks
-BD
-Bio-Rad
D or
- VersaTREK -I2A
I
-MAST
-BioMaxima
Media
M
-Oxoid
h
-Oxoid (Thermo Fisher)
-Rosco
t
-BBL (BD)
C
-Agricon Ricerche
S u
-bioMérieux
a
-Bio-Rad
E
-Liofilchem
-LIP/Fannin
Gunnar Kahlmeter, EUCAST 2019a r
Clinical trials
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
ib r
e L
I D or
CM t h
S a u
E Gunnar Kahlmeter, EUCAST 2019a r
r
Current (2019) ATU warnings:
ib
# Species Agent Breakpoints ATU Breakpoints ATU
≤/> (MIC) ≥/< (zone)
1 Enterobacterales Amoxicillin- 8/8 - 19/19 19-20
L
clavulanic
2 Enterobacterales Piperacillin- 8/16 16 20/17 17 - 19
e
tazobactam
3 Enterobacterales Ceftaroline 0.5/0.5 - 23/23 22 - 23
4 Enterobacterales Ciprofloxacin 0.25/0.5 0.5 25/22 22 - 24
5 Pseudomonas Piperacillin- 16/16 - 18/18 18 - 19
tazobactam
D or
6 Pseudomonas Ceftazidime- 8/8 17/17 16 - 17
I
aeruginosa avibactam
7 Pseudomonas Colistin 2/2 4 - -
aeruginosa
8 S. epidermidis Cefoxitin- - - 25/25 25 - 27
M
screen
h
9 S. aureus Ceftaroline 1/1 1 20/20 19 - 20
t
10 S. aureus Ceftobiprole 2/2 2 17/17 16 - 17
C
11 S. aureus Amikacin 8/16 16 18/16 15 - 19
12 H. influenzae (PBP3 Ampicillin 1/1 - 16/16 16 - 19
u
mutation)
S
13 H. influenzae (PBP3 Amoxicillin- 2/2 - 15/15 14 - 16
a
mutation) clavulanic
14 H. influenzae (PBP3 Piperacillin- 0.25/0.25 - 27/27 24 - 27
E
mutation) tazobactam
15 H. influenzae (PBP3 Several See
mutation) cephalosporins breakpoints
16 H. influenzae (PBP3 Imipenem 2/2 - 20/20 6 - 19
mutation)
Gunnar Kahlmeter, EUCAST 2019a r
r
Current (2019) ATU warnings:
ib
# Species Agent Breakpoints ATU Breakpoints ATU
≤/> (MIC) ≥/< (zone)
1 Enterobacterales Amoxicillin- 8/8 - 19/19 19-20
L
clavulanic
2 Enterobacterales Piperacillin- 8/16 16 20/17 17 - 19
e
tazobactam
3 Enterobacterales Ceftaroline 0.5/0.5 - 23/23 22 - 23
4 Enterobacterales Ciprofloxacin 0.25/0.5 0.5 25/22 22 - 24
5 Pseudomonas Piperacillin- 16/16 - 18/18 18 - 19
tazobactam
D or
6 Pseudomonas Ceftazidime- 8/8 17/17 16 - 17
I
aeruginosa avibactam
7 Pseudomonas Colistin 2/2 4 - -
aeruginosa
8 S. epidermidis Cefoxitin- - - 25/25 25 - 27
M
screen
h
9 S. aureus Ceftaroline 1/1 1 20/20 19 - 20
t
10 S. aureus Ceftobiprole 2/2 2 17/17 16 - 17
C
11 S. aureus Amikacin 8/16 16 18/16 15 - 19
12 H. influenzae (PBP3 Ampicillin 1/1 - 16/16 16 - 19
u
mutation)
S
13 H. influenzae (PBP3 Amoxicillin- 2/2 - 15/15 14 - 16
a
mutation) clavulanic
14 H. influenzae (PBP3 Piperacillin- 0.25/0.25 - 27/27 24 - 27
E
mutation) tazobactam
15 H. influenzae (PBP3 Several See
mutation) cephalosporins breakpoints
16 H. influenzae (PBP3 Imipenem 2/2 - 20/20 6 - 19
mutation)
Gunnar Kahlmeter, EUCAST 2019You can also read