Hope for Children with Orphan Liver Diseases - Through Bile Acid Modulation August 2020 - Investors
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Cautionary Note Regarding Forward-Looking Statements
This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements, other than historical facts,
regarding, among other things: the plans for, or progress, scope, cost, initiation, duration, enrollment, results or timing for availability of results of, development of odevixibat or any of our other product
candidates or programs, including regarding expectations regarding the impact of COVID-19 on our business and our ability to adapt our approach as appropriate; the Phase 3 clinical program for
odevixibat in patients with progressive familial intrahepatic cholestasis (PFIC), the pivotal trial for odevixibat in biliary atresia, the planned pivotal trial for odevixibat in Alagille syndrome and a Phase 2 trial
for elobixibat being conducted by EA Pharma in Japan; the target indication(s) for development or approval, the size, design, population, location, conduct, cost, objective, enrollment, duration or
endpoints of any clinical trial, or the timing for initiation or completion of or availability or reporting of results from any clinical trial, including the Phase 3 PFIC trial for odevixibat, and the long-term open-
label extension study, the pivotal trial for odevixibat in biliary atresia, the planned pivotal trial for odevixibat in Alagille syndrome, for submission of any regulatory filing, or for discussions with regulatory
authorities; the timing of and our ability to obtain and maintain regulatory approval of any of our product candidates and any related restrictions, limitations, or warnings in the label of any approved
product candidates; the timing for commercialization of any of our product candidates, if approved; the size of the PFIC population, the biliary atresia population or any other disease population for
indications that may be targeted by Albireo; the potential benefits or competitive position of odevixibat or any other Albireo product candidate or program or the commercial opportunity in any target
indication; the potential benefits of a rare pediatric disease designation; the potential benefits of a fast track designation; the potential benefits of orphan drug designation; the pricing of odevixibat if
approved; any action by, or decision of, EA Pharma concerning elobixibat or our business relationship; the duration of our cash runway; our future operations, financial position, revenues, costs, expenses,
uses of cash, capital requirements or our need for additional financing; or our strategies, prospects, beliefs, intentions, plans, expectations, forecasts or objectives. Words such as “anticipates,” “believes,”
“plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions sometimes identify forward-looking
statements.
Any forward-looking statement involves known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially
from those expressed or implied by such forward-looking statement, and, therefore, investors are cautioned not to place undue reliance on any forward-looking statement. These factors include, but are
not limited to: negative impacts of the COVID-19 pandemic, including on manufacturing, supply, conduct or initiation of clinical trials, or other aspects of our business; the potential availability of
odevixibat through the EAP, whether the United States Food and Drug Administration (FDA) continues to allow odevixibat to be administered through the Expanded Access Program (EAP), whether
favorable findings from clinical trials of odevixibat to date, including findings in indications other than PFIC, will be predictive of results from the trials comprising the Phase 3 PFIC program or any other
clinical trials of odevixibat; whether either or both of the FDA and European Medicines Agency (EMA) will determine that the primary endpoint for their respective evaluations and treatment duration of the
double-blind Phase 3 trial in patients with PFIC are sufficient, even if the primary endpoint is met with statistical significance, to support approval of odevixibat in the United States or the European Union,
to treat PFIC, a symptom of PFIC, a specific PFIC subtype(s) or otherwise; the outcome and interpretation by regulatory authorities of the ongoing third-party study pooling and analyzing of long-term PFIC
patient data; the timing for initiation or completion of, or for availability of data from, clinical trials of odevixibat, including the trials comprising the Phase 3 PFIC program, the pivotal program in biliary
atresia or the planned pivotal program in Alagille syndrome, and the outcomes of such trials; Albireo’s ability to obtain coverage, pricing or reimbursement for approved products in the United
States or European Union; delays or other challenges in the recruitment of patients for, or the conduct of, the double-blind Phase 3 trial of odevixibat; whether odevixibat will meet the criteria to receive a
pediatric priority review voucher when applicable; the competitive environment and commercial opportunity for a potential treatment for PFIC or other orphan pediatric cholestatic liver diseases; the
medical benefit that may be derived from odevixibat, elobixibat, A3384 or any of our other product candidates; the extent to which our agreement for elobixibat with EA Pharma generates future
nondilutive income; the significant control or influence that EA Pharma has over the commercialization of elobixibat in Japan and the development and commercialization of elobixibat in EA Pharma’s other
licensed territories; our ability to protect and expand our intellectual property; the timing and success of submission, acceptance and approval of regulatory filings; and our critical accounting policies.
These and other risks and uncertainties that we face are described in our most recent Annual Report on Form 10-K and in other filings that we make or have made with the Securities and Exchange
Commission. In addition, market and industry statistics contained in this presentation are based on information available to us that we believe to be reliable but have not independently verified.
All forward-looking statements speak only as of the date this presentation is made and should not be relied upon as representing our views as of any date after this presentation is
made. We specifically disclaim any obligation to update any forward-looking statement, except as required by applicable law. “Albireo” is a trademark of Albireo AB. All other trademarks,
service marks, service marks, trade names, logos and brand names identified in this presentation are the properties of their respective owners.
2 ©2020 Albireo Pharma, Inc. All rights reservedAlbireo: Innovative Science + Deep Pipeline
+ Well Capitalized
STRONG ▪ More than a decade of leadership in bile acid modulation
BASIC ▪ World’s first regulatory approval for IBATi therapy (elobixibat)
SCIENCE
ORPHAN
PEDIATRIC ▪ Odevixibat (IBATi) wholly owned, oral QD capsule/sprinkle with MOU patent
LIVER LEAD through 2031/34*, orphan desigs., PRIME, PIP, fast track and PRV eligibility
ASSET ▪ Adult liver and bile acid malabsorption programs
SOLID ▪ Nasdaq listed as ALBO; 15M outstanding shares as of June 30, 2020
FINANCIAL ▪ $152M cash and cash equivalents as of June 30, 2020
POSITION ▪ Cash into the beginning of 2022; past planned odevixibat approval/launch
*Natural expiry/with potential patent term extension (PTE)
3 ©2020 Albireo Pharma, Inc. All rights reservedManagement Team With Deep Biotech & Pharma Experience
Ron Cooper Jan Mattsson, PhD
President and CEO Chief Scientific Officer
(Co-Founder)
Bristol-Myers Squibb
(President of Europe) AstraZeneca
Pat Horn, MD, PhD Simon Harford
Chief Medical Officer Chief Financial Officer
Parexel, GlaxoSmithKline,
Orphan Technologies, Eli Lilly
Dyax, Tetraphase, Abbott
Pamela Stephenson Martha Carter
Chief Commercial Officer Chief Regulatory Officer
Vertex, Pfizer Aegerion, Proteon, Trine
Michelle Graham Jason Duncan
Chief Human Resources Officer Chief Legal Officer
and General Counsel
TESARO, Parexel, Integer, Stallergenes Greer, Sobi,
Bausch + Lomb, Bristol-Myers Squibb
EMD Serono
4 ©2020 Albireo Pharma, Inc. All rights reservedA Robust Pipeline Targeting Liver and GI
Diseases/Disorders
PRECLINICAL PHASE 1 PHASE 2 PHASE 3 APPROVED
Commercialization
PFIC
Independent
Odevixibat
Planned
Biliary Atresia
Pediatric Liver
Diseases Alagille Syndrome
Other Cholestatic
Chronic Approved in Japan/Partnered with EA Pharma
Elobixibat
Constipation
Commercialization
Planned Partner
Adult Liver
Lead Candidate
Diseases
Bile Acid
Undisclosed
Modulators
Bile Acid
A3384
Malabsorption
5 ©2020 Albireo Pharma, Inc. All rights reservedDelivering on Our Plan as a Public Company
Elobixibat
Ph3 Odevixibat
Japan
Biliary Atresia
Approved
Pivotal Start
Ph3
Ph3 Ph3
Ph2 Odevixibat Odevixibat PFIC
Elobixibat Odevixibat
Odevixibat Site Ph.3
Japan Ph3 PFIC Pivotal
Results Initiation Last Patient
Results Start
Completed Visit Completed
2016 2017 2018 2019 2020
NASDAQ Equity Raise ATM Equity
Equity Raise
Listing ~$50M Financing Raise
~$100M
~$30M ~$21M ~$43M
Legacy Asset
Elobixibat Sale Royalty
Royalty
Milestone ~$4.5M Monetization
Monetization
Payment ~$45M
~$15M
~$8M
Elobixibat
Debt Facility
Approval PRV
$10M
Milestone Eligibility
Payment Odevixibat
~$11M
6 ©2020 Albireo Pharma, Inc. All rights reservedMultiple Planned Milestones
1H’20 2H’20 1H’21 2H’21 2022
PFIC PEDFIC 1: Phase 3 topline data Mid 2020 *Last patient, last visit achieved*
PFIC PEDFIC 2 rollover and expanded cohort Open label
Biliary atresia pivotal program 1H’20 Initiation 1H’21 Full site activation
Alagille syndrome pivotal program EOY ‘20 Initiation
PFIC approval and launch 2H’21
Lead Candidate Adult Liver Disease (MOA undisclosed) IND-enabling studies
Novel bile acid modulators
7 ©2020 Albireo Pharma, Inc. All rights reservedMany Diseases with Cholestasis of the Liver
Cystic Fibrosis-
Intrahepatic Associated Liver Disease
Progressive Familial Cholestasis of
Intrahepatic Pregnancy Primary Biliary
Cholestasis (PFIC) Cholangitis AIDS
Cholangiopathy
Drug-Induced
Cholestasis
Malignancy of Bile Ducts
Biliary Atresia
IG4-associated cholangitis
Alagille Syndrome
Low Phospholipid-
Biliary Associated Cholestasis
Primary Sclerosing Cholangitis Strictures
9 ©2020 Albireo Pharma, Inc. All rights reservedPotential Target Indications
~30,000-40,000* patients in the U.S. and EU alone who are lacking an
approved pharmacological treatment
40
Alagille 3-5K
35 Genetic disorder, paucity of bile ducts
30 PFIC
8-10K
Genetic disorders with bile acid build-up in liver
25
Thousands
Pediatric
20 PSC
8-10K Inflammation and scarring of bile ducts
15
Biliary Blocked or absent large bile ducts
10
Atresia
15-20K
5
*Estimate derived from literature, primary market research and modeling. Forecast
0 estimates do not include other regional opportunities, such as Saudi Arabia, Turkey,
Bile Acid-Associated Asia, LATAM.
Cholestatic Liver Diseases
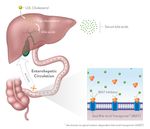
10 ©2019 Albireo Pharma, Inc. All rights reserved.What Is PFIC?
Genetic Disease
Presentation Survival
Disorder Progression
Multiple genes, Inflammation Almost no patients
Age ~1-2 similar symptoms Fibrosis survive beyond age 20
Cirrhosis without surgical
Cholestatic/ Death diversion or liver
Pruritic transplant*
*Pawlikowska 2010
11 ©2020 Albireo Pharma, Inc. All rights reservedInadequate Treatment Options for PFIC
Off-Label Medications PEBD Surgery Liver Transplantation
(partial external biliary diversion)1
UDCA
0 1 2
Time Post PEBD ( Years)
Seeking symptomatic relief Bile acid and pruritus reductions Limited timely organ availability
UDCA, rifampicin, cholestyramine … Undesirable external stoma bag Need for lifelong immunosuppression
Morbidity and disease recurrence
1Yang, et al. J Pediatr Gastroenterol Nutr 2009
12 ©2020 Albireo Pharma, Inc. All rights reservedKennedie’s Story
Diagnosis Insatiable Pruritis Life Post-Transplant
✓ Failure to thrive “We did what we could. ✓ Urgent need for liver transplant
✓ Unexplained seizure, brain bleed Nothing could comfort her. ✓ Lengthy hospitalization
✓ Undetectable levels of Vitamins Nothing helps the ✓ Various setbacks
A, D, E, K
insatiable itching.” ✓ Compromised immune system
✓ PFIC 2 diagnosis at 6 months
✓ Requires daily immunosuppressive
-Emily, Kennedie’s mother medications
For more patient stories, visit www.pficvoices.com/videos
13 ©2020 Albireo Pharma, Inc. All rights reservedOdevixibat: A Profile Potentially Suitable for Pediatric Use
▪ Once-Daily Dosing
▪ Oral Capsule or Sprinkles
▪ Minimal Systemic Exposure
▪ Favorable Tolerability Profile*
*In Phase 2 clinical trial
14 ©2020 Albireo Pharma, Inc. All rights reservedNAPPED Natural History Data
Provide Strong Rationale for IBATi
NAPPED: Natural Course and Prognosis of PFIC and Effect of Biliary Diversion
PFIC2 Native Liver PFIC1 Native Liver
Survival Improvement* Survival Improvement **
% Of Patients With Native Liver
% Of Patients With Native Liver
P=0.03 Years After Diversion
P=0.001 Years After Diversion
Improved native liver survival does not require bile acid normalization
*Van Wessel et al. 10.1016/j.hep.2020.02.007, Would be 100%, but one patient died due
to complications of multiple PEBD surgeries ** Van Wessel Espghan 2019
15 .
©2020 Albireo Pharma, Inc. All rights reservedOdevixibat: Phase 2 Trial in Pediatric Cholestatic Liver Disease
Odevixibat doses evaluated (µg/kg), 4 weeks
10 30 60 100 200
PFIC, Biliary Atresia, Alagille Syndrome, Intrahepatic Cholestasis Patients
▪ Open-label, dose-finding trial
▪ Primary endpoints: TESAEs and serum bile acid change
▪ Baseline – single test dose – 2-wk washout – 4-wk treatment
▪ Trial initially designed with a maximum dose of 300 µg/kg
▪ N=24 (20 unique + 4 retreated)
Oral late breaker EASL’17/Presidential Poster of Distinction AASLD’17
Odevixibat
Phase 2
Pediatric
Trial
16 ©2020 Albireo Pharma, Inc. All rights reservedPrimary Efficacy Endpoint:
Reduction Demonstrated in Serum Bile Acids
All Patients PFIC Patients Only*
0
-10
Mean (SEM) % change from
baseline in serum bile acids
-20 -31
-30
-48 -51
-40 -56
-63
-50
-60
-70 *
-80 Bars illustrate Standard Error of the Mean
**
-90
Phase 2 trial was an open-label, dose-finding trial of PFIC, biliary atresia, * Excludes PFIC patient with no BSEP function and 17-year-old PFIC
Alagille syndrome, intrahepatic cholestasis patients for four weeks. patient with low baseline sBA. Neither meet inclusion criteria for Phase 3
Odevixibat Primary endpoints: TESAEs and serum bile acid change trial.
Phase 2 N=24 (20 unique + 4 retreated) in five cohorts
Pediatric *Excludes PFIC patient with no BSEP function.
Trial **Excludes 17-year-old PFIC patient with low baseline sBA.
Neither meet inclusion criteria for Phase 3 trial.
17 ©2020 Albireo Pharma, Inc. All rights reservedSerum Bile Acids in Alagille Syndrome
and Biliary Atresia Patients
Alagille Syndrome Biliary Atresia
Baseline µM 260 116 338 26 121 564 43 136 132
0
-14
-20
Serum Bile -39
Acids -40
-52 -51
% reduction -57 -58
from baseline
-60
-80
-92
10-200 ug/kg dose, 4 weeks of treatment 30 ug/kg, 4 weeks of treatment
-100
Odevixibat
1 2 3 4 5 6 1 2 3
Phase 2
Pediatric
Trial Patient
18 ©2020 Albireo Pharma, Inc. All rights reservedStatistical Correlation Supports Link Between Reductions
of Serum Bile Acids and Pruritus
VAS-Itcha Whitington (itch)b
ap=0.008, r=0.54, n=23.
bp=0.004, r=0.58, n=23.
cp=0.006, r=0.57, n=22.
dp=0.005, r=0.57, n=22.
PO-SCORAD (itch)c PO-SCORAD (sleep)d
Odevixibat
Phase 2
Pediatric
Trial
nFavorable Tolerability Profile in Trial
▪ All patients completed treatment; no evidence of diarrhea during 4-week treatment period
▪ No AEs related to treatment during 4-week treatment period
• Most common AEs: pyrexia, ear infections (12.5%)
▪ No SAEs designated as treatment related (2 deemed unrelated)
▪ Decision made not to dose escalate above 200 µg/kg
• Some transaminase elevations at 200 µg/kg
Odevixibat
Phase 2
Pediatric
Trial
20 ©2020 Albireo Pharma, Inc. All rights reservedPEDFIC 1&2: Phase 3 PFIC Program Summary
Pediatric PFIC (PEDFIC)
24-Week Treatment
Odevixibat
Endpoints
40 µg/kg/day
N~20
FDA
PEDFIC 2
• Assessment of change Rollover cohort
in pruritus extension trial
62 Subjects
Odevixibat EMA
Target 60 R 120 µg/kg/day • Serum bile acid
Oral capsule/sprinkle
Once daily
N~20 responder rate (reach PEDFIC 2
≤70 μmol/L or a Expanded cohort
reduction of 70%) non-PEDFIC 1 eligible
Key Inclusion Criteria: Placebo FDA/EMA: Single Pivotal
Diagnosis of PFIC1 or 2 N~20
Sufficient to Support
Confirmed BSEP activity
NDA/MAA Filings
Serum bile acids ≥100 μmol/L
Pruritus ≥2 on 0-4 scale Double-Blind, Randomized, Placebo-Controlled
Trial to Demonstrate Efficacy and Safety of
Odevixibat in Children with PFIC
Launched Expanded Access Program
U.S., Europe, Canada, and Australia
21 ©2020 Albireo Pharma, Inc. All rights reservedProprietary PRUcisionTM Pruritus Measurement Tool
Rigorously Designed for our Phase 3 Target Population
1a. How bad was your worst itching since you
went to bed last night?
PRO+ObsRO: 0-4 scales
• Each response distinguished by pictures, words, numbers and colors
• Tested with both patients and caregivers
• Multiple interactions with FDA in development of the tool
22 ©2020 Albireo Pharma, Inc. All rights reservedPlanning For Success
Manufacturing Expand Pt. Population Go to Market
▪ Agreed elements of CMC plan w/FDA ▪ Initiated biliary atresia pivotal trial ▪ KOL engagement
▪ Planned commercial formulation in Ph3 ▪ Plan to initiate Alagille pivotal trial ▪ Pricing and access planning
▪ Registration batches on stability ▪ Evaluate additional indications ▪ Patient support program build
23 ©2020 Albireo Pharma, Inc. All rights reservedExpansion Opportunity: Biliary Atresia
Presentation Cause Treatment Disease Progression
Age Absence of Kasai (HPE) ~50% of patients
~2 wk-3 mos. bile ducts have liver transplant
in first 2 years1
Failure to thrive Surgery may Transplant is definitive
Acholic stools restore bile flow treatment
Jaundice
#1 Cause of Pediatric Liver Transplants
Estimated Prevalence 15-20K (U.S./EU)
1Data on file;2Lykavieris et al. Hepatology, 2005
24 ©2020 Albireo Pharma, Inc. All rights reservedBile Acids: Significant Impact in Biliary Atresia
Lower sBA Correlated Improved Liver Markers Correlated
300 With Improved NLS1 With Lower Serum Bile Acids2
Median Serum Bile Acid
Concentration (μmol/L)
100 Low Bile AcidsL(≤40
o w μM)
tie nPatients
B ile A c id s (
High Bile Acids (>40 μM)
T w o -Y e a r O u tc o m e s
227
200 80 H ig h B ile a c id s
ts )
Death or Liver Transplant
(% o f p a of
Kasai 60
Surgery 139
Percentage
100 40
110
20
59 39
n= 516 Alive Native Liver
0
0
A ALT
LT GGT P l a te l e ts S p le e n
Baseline
Baseline 6Month 6
Months Last visit*
2 years GGT Platelets Spleen
( 4 0(≤40
U /L ) ( 55 U /L ) ( 1 5 0 / L ) ( 2 c m b e l o w
(≤55 (≥150/ηL) c(≤2 cm below
o sta l r e g i o n )
U/L) U/L) costal region)
sBA Reduction Correlated With Sustained Improvements Post-Kasai (HPE) Over 2 Yrs.
ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; HPE, hepatoportoenterostomy; sBA, serum bile acids.
1. Data on file; 2. Harpavat et al. Hepatology. 2018;68(suppl 1):85A–86A.
25 ©2020 Albireo Pharma, Inc. All rights reservedBOLD: Precedent-Setting Biliary Atresia Pivotal Trial
Biliary Atresia and the Use of Odevixibat in Treating Liver Disease (BOLD)
24-Month Treatment
Odevixibat
120 µg/kg/day
N= 100 Primary Endpoint
~200 Subjects
Post-Kasai HPE Proportion of patients who Rollover cohort
Oral capsule/sprinkle
R are alive and have not Extension Trial
Once daily undergone a liver transplant
Placebo
N= 100
FDA/EMA: Single Pivotal
Key Inclusion Criteria: Sufficient to Support Filing
Clinical Diagnosis of BA
Age at Kasai HPE ≤ 90 days
Randomized within 3 weeks Double-Blind, Randomized, Placebo-Controlled Trial
to Evaluate the Efficacy and Safety of Odevixibat in
Children with BA who have undergone Kasai HPE
~70 global sites will be initiated
26 ©2020 Albireo Pharma, Inc. All rights reservedExpansion Opportunity: Alagille Syndrome
Genetic Disease
Presentation Impact
Disorder Progression
Age Autosomal Paucity of Many patients
~4-12 Mos. dominant bile ducts may need a liver
transplant
Multiple Multiple
Symptoms Organ Impact Disease can stabilize
?
Initiation of Planned Pivotal Trial by EOY 2020
FDA and EMA Agreement on Protocol Design
▪ Estimated prevalence 3-5K (U.S./EU)
▪ Orphan designations received in U.S. and EU
27 ©2020 Albireo Pharma, Inc. All rights reservedOdevixibat: Expanding Development Across Pediatric CLDs
Pediatric
Liver Disease
Other Rare Franchise
CLDs
Alagille
Biliary Other
Atresia Indications
Planned
pivotal trial
initiation by
EOY 2020
PFIC Pivotal trial
initiated H1 2020
Mid-2020 topline
Ph.3 results
anticipated
28 ©2020 Albireo Pharma, Inc. All rights reservedCommercialization Strategy
U.S. Launch EU Launch RoW Strategy
Identify Patients Focused Medical Robust Country
Presence Prioritization
Competitive Strong Market Strong Local
Profile Access Partners
Drive Access to Flexible Commercial
Accelerate Uptake Operations
29 ©2020 Albireo Pharma, Inc. All rights reservedDRAFT
Odevixibat Go-to-Market Plan
PFIC Ph3 Results U.S./EU Launch
2019 2020 2021
Completed Hire Field
Commercial Account Mapping Hire and Train Field Teams
Management
Activities Doctors
Key Data Presentations & Publications
Commercial
Hires
Physician, Hire Account Scientific Finalize
Patient & Value Story and Economic Models
Team Exchange Pricing
Payer Market
Research Access
Product Supply
Early Access Programs & Distribution Planning
PFIC Voices Readiness
& Advocacy
Market Access
Strategy Hire and Train Case
Develop Patient Support Program
Global Market Managers
Prioritization Patients
Brand Name Expand PFIC Awareness Campaign and Ongoing Advocacy
30 ©2020 Albireo Pharma, Inc. All rights reservedUnencumbered Global Rights and Strong Patent Estate
▪ Method of Use Patent Expiration 2034*
• 3 patents, 10+ claims targeted to PFIC
• Multiple Orange-Book listable patents for PFIC and CLDs
▪ Orphan exclusivity in the U.S. (7 yrs.) and EU (10+2yrs.)
▪ Composition of Matter 2025*
Strength of Method-of-Use Patents
WEAK STRONG
Unmet Need New Use Pediatric and New Chemical
Formulations
Orphan Entity (NCE)
Routes of Administration
Population
*with PTE and pediatric extensions
31 ©2020 Albireo Pharma, Inc. All rights reservedHigh Unmet Need and Compelling Opportunity
▪ Pediatric Cholestasis: orphan indications with no approved drug
▪ PEBD: strong clinical rationale for potential benefit of IBAT inhibition
▪ Odevixibat: serum bile acids, pruritus, low diarrhea in pediatric Ph.2 trial
▪ Three Pivotal Programs: PFIC, biliary atresia, Alagille syndrome
▪ Exclusivity Position: orphan drug designations (U.S.-7/EU-12* years);
COM 2022/25**; MOU for specified cholestatic liver diseases, 2031/34**
▪ Attractive P&L: modest commercial infrastructure required, few target Rx’ers
*Assumes execution of agreed PIP **Natural expiry/with potential PTE
32 ©2020 Albireo Pharma, Inc. All rights reservedMultiple Upcoming Milestones Anticipated Odevixibat PFIC: PEDFIC 1 Phase 3 topline data Mid 2020 Odevixibat Alagille syndrome: Initiate planned pivotal program EOY 2020 Elobixibat NASH: Japan Phase 2 trial topline data EOY 2020/1Q’21 Odevixibat PFIC: Potential approval and launch 2H 2021 33 ©2020 Albireo Pharma, Inc. All rights reserved
Hope for Children with Orphan Liver Diseases Through Bile Acid Modulation August 2020
You can also read