The bouquet of grapevine (Vitisvinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
The bouquet of grapevine (Vitis vinifera L. cv. Cabernet
Sauvignon) flowers arises from the biosynthesis
of sesquiterpene volatiles in pollen grains
Diane M. Martina,b, Omid Touba,b, Angela Chiangb, Bernard C. Loa,b, Sebastian Ohsea,b, Steven T. Lundb,
and Jörg Bohlmanna,b,1
aMichael Smith Laboratories and bWine Research Centre, University of British Columbia, Vancouver, BC, Canada V6T 1Z4
Edited by Richard A. Dixon, The Samuel Roberts Noble Foundation, Ardmore, OK, and approved March 11, 2009 (received for review February 6, 2009)
Terpenoid volatiles are important information molecules that en-
able pollinators to locate flowers and may protect reproductive
A B C
tissues against pathogens or herbivores. Inflorescences of grape-
vine (Vitis vinifera L.) are composed of tiny green flowers that
produce an abundance of sesquiterpenoid volatiles. We demon-
strate that male flower parts of grapevines are responsible for
sesquiterpenoid floral scent formation. We describe temporal and
spatial patterns of biosynthesis and release of floral volatiles
throughout the blooming of V. vinifera L. cv. Cabernet Sauvignon.
The biosynthesis of sesquiterpene volatiles, which are emitted D E F
with a light-dependent diurnal pattern early in the morning at
fs
prebloom and bloom, is localized to anthers and, more specifically,
within the developing pollen grains. Valencene synthase (VvValCS) b
enzyme activity, which produces the major sesquiterpene volatiles
of grapevine flowers, is present in anthers. VvValCS transcripts are
most abundant in flowers at prebloom stages. Western blot anal-
ysis identified VvValCS protein in anthers, and in situ immunola-
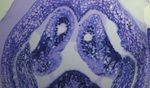
beling located VvValCS protein in pollen grains during bloom. Fig. 1. Flower development on rooted cuttings of grapevine (V. vinifera L.
cv. Cabernet Sauvignon) used for headspace volatile collection. (A) Flower
Histochemical staining, as well as immunolabeling analysis by
panicle (top node) of a rooted cane inside the volatile collection chamber. The
fluorescent microscopy and transmission electron microscopy, in- lateral vine (lower node) is below the chamber (not shown). (B) Individual
dicated that VvValCS localizes close to lipid bodies within the flowers clockwise from top right at stage VI, just opening, and in bloom. A
maturing microspore. penny is used as a scale reference. (C) Stage IV flowers; the flowers are
separated from one another. (D) Stage V flowers; the flowers are lengthened.
anthers 兩 floral scent 兩 flower development 兩 sesquiterpenes 兩 (E) Stage VI flowers; just before bloom, when the flowers are lengthened. (F)
terpenoid biosynthesis Flowers at bloom (b) and at fruit set (fs) several days after bloom are identified
PLANT BIOLOGY
by a swelling of the pistil.
A lthough the release of volatiles, including aliphatics, terpe-
noids, and phenylpropanoids, from anthers and/or pollen
has been reported (1), the molecular mechanisms and localiza-
Clarkia breweri, snapdragon, tobacco, Arabidopsis, roses) pro-
vides the bulk of information regarding the regulation and
tion of pollen volatile formation remain uncertain. Pollen vola- biosynthesis of flower volatiles, with a focus of floral scent
tiles serve as attractants for pollinators and also may function to emissions from the petals and stigma of showy flowers (12).
deter herbivores and defend against pathogens (2). They can be Transcript profiling in Arabidopsis and Magnolia grandiflora
distinct from the scents of other floral organs and may decrease
found the expression of sesquiterpene synthases in anthers (13,
after pollination, thereby advertising the pollen of unvisited
14), suggesting a role for male flower organs in the biosynthesis
flowers to pollinators (1, 3). Pollen volatiles are thought to be
of floral volatiles.
localized to the pollenkitt, a waxy substance localized to the
Here we describe the qualitative and quantitative composition
grooves in the exine, which provides protection against patho-
gens, desiccation, or UV light and assists in pollen–pistil inter- and the spatial, temporal, and developmental patterns of the
actions (1, 4). Various nonvolatile compounds of the pollenkitt biosynthesis and release of Vitis vinifera L. cv. Cabernet Sau-
and sporopollenin are deposited on the surface of microspores vignon floral sesquiterpene volatiles. Anthers are identified as
by tapetum cells (4). Although it has been postulated that the the floral organ with the highest levels of sesquiterpene volatiles
tapetum also produces volatiles (1, 5), this has not been estab- immediately before bloom and at bloom. These volatiles seem to
lished experimentally.
Grapevines produce dense panicles of small flowers with tiny
Author contributions: D.M.M. and J.B. designed research; D.M.M., O.T., A.C., B.C.L., and
petals fused into a cap (Fig. 1). Most cultivated grapevines have S.O. performed research; S.T.L. contributed new reagents/analytic tools; D.M.M., O.T., A.C.,
perfect hermaphroditic flowers and are thought to be at least B.C.L., and S.O. analyzed data; and D.M.M. and J.B. wrote the paper.
partially autogamous or cleistogamous (6), whereas their wild The authors declare no conflict of interest.
relatives are dioecious and have functionally pistillate or stami- This article is a PNAS Direct Submission.
nate flowers that require insect pollinators for fertilization (7, 8). Data deposition: The sequence reported in this paper has been deposited in the GenBank
The composition of floral volatiles of several cultivated grape- database (accession no. FJ696653).
Downloaded by guest on November 4, 2021
vine varieties has been described (9–11), but little else is known 1To whom correspondence should be addressed. E-mail: bohlmann@msl.ubc.ca.
about the molecular biochemistry of grapevine floral scent. This article contains supporting information online at www.pnas.org/cgi/content/full/
Research over the last 15 years in only a few plant species (e.g., 0901387106/DCSupplemental.
www.pnas.org兾cgi兾doi兾10.1073兾pnas.0901387106 PNAS 兩 April 28, 2009 兩 vol. 106 兩 no. 17 兩 7245–7250be localized initially within the pollen grain and later within and
outside of the pollen grain. The sesquiterpene synthase VvValCS
A 1800
produces the majority of sesquiterpene pollen volatiles in the 1500
male flower parts. This work provides fundamental new infor-
pg volatiles released
mation about the biosynthesis and localization of terpenoid
/ flower / hour
1200
volatiles in anthers and the developing pollen grains.
Results 900
`
Grapevine Flowers Release Bursts of Sesquiterpene Volatiles in the
Morning. We collected flower volatiles prebloom and throughout 600
bloom from inflorescences of the first or second node from the
top of rooted grapevine (V. vinifera L. cv. Cabernet Sauvignon)
300
cuttings (Fig. 1 A). Leaves from a lateral shoot of a lower node
were kept outside of the headspace collection system. During
bloom, volatiles were emitted with a maximum of up to 1,047 (⫾ 0
6 7 8 9 10 11 16 21 6 7 8 9 10 11 16 21 6 7 8 9 10 11 16 21 6 7 8 9 10 11 16 21 6 7 8 9 10
504) pg/flower/h, primarily in the morning between 0600 and Collection Start (hr)
1100 hours, which coincided with the beginning of the light
period [Fig. 2A and supporting information (SI) Fig. S1 A]. The B 250
relatively large variation in the amounts of emissions detected for
a given time point likely is due to the fact that opening of flowers
200
within a cluster is not synchronized and varies between clusters.
pg volatiles released
The release of volatiles between 0600 and 1100 hours remained
elevated over a period of 3–5 days while individual flowers of a
/ flower / hour
150
given cluster continued to open. The largest amount of volatiles
was released during two 1-h sampling periods between 0600 and
0800 hours (Fig. 2 A). During the time of maximum volatile 100
release, sesquiterpenes composed up to 86% of total emissions,
whereas aliphatic compounds composed up to 16% of total
50
emissions (Fig. S1B, Table S1). The sesquiterpenes (⫹)-
valencene, ⌭,⌭-␣-farnesene, and (-)-7-epi-␣-selinene were the
most prominent, with up to 60% of all volatiles released at the 0
height of emissions (Table S1). The relative proportion of 6 11 16 21 6 11 16 21 6 11 16 21 6 11 16 21 6 11 16 21 6 11
aliphatic compounds increased during the later part of the day Collection Start (hr)
and throughout the night (Fig. S1B). Absolute emissions of
either class of compounds dropped to near-background levels at C 300
night, suggesting that their release is influenced by circadian
250
rhythm, light, and/or temperature.
pg volatiles released
No increase in volatile emission was seen before the onset of
the light period between 0500 and 0600 hours (data not shown),
/ flower / hour
200
and the rhythmic pattern of diurnal emission continued when
temperature was kept constant at 22 °C during the light–dark 150
cycles (Fig. S1C). To test whether volatile emission is controlled
by a circadian rhythm, volatiles were measured over extended 100
dark periods and light periods (Fig. 2 B and C) beginning after
the first day of bloom. The diurnal rhythm of emission was still
50
present after 4 days without light (Fig. 2B). Conversely, the
pattern of peak emission between 0600 and 1100 hours was lost
at constant light (Fig. 2C). These results suggest that light, but 0
6 11 16 21 6 11 16 21 6 11 16 21 6 11 16 21 6 11 16 21 6 11
not temperature, is a critical factor for the rhythmic diurnal Collection Start (hr)
emission of volatiles from grapevine flowers, but conditions of
constant light disturb this endogenous circadian rhythm. Fig. 2. Time course of volatile emissions from grapevine flowers at bloom. Data
represent the amounts of volatiles detected by GCMS. Gray bars illustrate the
Volatiles Accumulate in Prebloom Anthers Before Emission at Bloom. period of darkness for each experiment. (A) Time course of volatile emissions
Over a 4-day period, we examined whether the rate of volatile divided into hourly segments between 0600 and 1100 hours. Bars represent 2– 6
replicates ⫹ SEM. (B) Volatile emissions during constant darkness after the first
emission correlated with the number of flowers open within a
day of blooming. Bars represent 5 replicates ⫹ SEM. (C) Volatile emissions during
flower cluster (Table S2). Flowers were considered open when constant light conditions. Bars represent 3 replicates ⫹ SEM.
the caps had fallen off (Fig. 1B). No substantial volatile release
was detected on the first day, when most flowers were closed.
The maximum volatile emission was detected on day 3, when
50% of the flowers were visibly open. Additional flowers may stages IV–VI (Fig. 1 C–E), at bloom, and at fruit set (Fig. 1F).
have been already partially open at this time, allowing the release Flowers were dissected into anthers, caps, and pistils, and the
of volatiles before the caps had fallen off, because opening of the individual flower parts were extracted in pentane. Most of the
cleistogamous flowers of cultivated grapevine often is caused by extractable volatile compounds were present in the anthers
anthers dehiscing with incomplete loss of caps (15). during prebloom stages V and VI and at bloom (Fig. 3A). In
To further investigate developmental and spatial patterns of contrast, very low amounts of the same compounds were found
in other flower parts or in anthers at stage IV and at fruit set.
Downloaded by guest on November 4, 2021
grapevine floral scent formation, we analyzed individual flow-
ering stages defined according to Gribaudo et al. (16) based on Sesquiterpenes composed a substantial portion of the total
visual appearance. We used flowers in prebloom development extractable compounds from anthers during prebloom stages V
7246 兩 www.pnas.org兾cgi兾doi兾10.1073兾pnas.0901387106 Martin et al.A 16
14
Anthers Total
Pistils Total
Caps total
µg compounds/flower
12
10
8
6
D E
4
2
0
IV V VI Bloom Fruit Set
Stage of Flower Development
B 8 sesquiterpenes
µg compounds/flower
7 diterpenes
others
6
5
4 F
3
7 9
2
10
Detector R es pons e
H
1
0
8
IV V VI Bloom Fruit Set 1 6
2-5 Anthers S tage V P entane extracts
Stage of Flower Development
V olatile C ollection 0800 - 0900
C 14
Sesquiterpenes VvValCS
µg compounds/flower
GeranylLinalool
12 All Compounds
10
19 20 21 22 23 24 25 26 27 28 29
8
Time (min)
6
4
2
0
Chloro:MeOH Pentane Chloro:MeOH Pentane
Stage V Stage VI
Fig. 3. GCMS analysis of volatile compounds extracted from grapevine flower parts. (A) Total pentane-extractable compounds present in anthers, pistils, and
caps. Bars represent 4 independent replicate extractions ⫹ SEM, with each replicate comprising 10 individual flowers per stage. (B) Pentane extracts from anthers
analyzed by type of compound present: sesquiterpenes, diterpenes, or aliphatics. Bars represent 4 replicates ⫹ SEM. (C) Quantitative analysis of the pollen
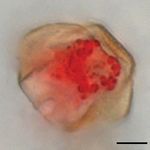
pentane and chloroform/methanol extracts from stage V and VI flowers. Bars represent 4 replicates ⫹ SEM. (D) Light microscopy image of a stage VI anther stained
with Oil Red O. (Scale bar ⫽ 100 m.) (E) High-magnification image of a pollen grain showing abundant lipid bodies stained with Oil Red O inside the pollen.
(Scale bar ⫽ 5 m.) (F) GCMS total ion chromatogram of the products from VvValCS enzyme (solid black line) compared with the anther extracts (solid gray line)
and the flower volatile emissions collected on a day of peak release (dashed line). Compounds are as follows: ⌭--caryophyllene (1), spirolepechinene (2)*,
␣-humulene (3), unknown sesquiterpene (4), ⌭--farnesene (5), selina-4,11-diene* (6), (⫹)-valencene (7), tridecanone/cis-␣-bergomatene (8), ⌭,⌭-␣-farnesene (9),
PLANT BIOLOGY
and (–)-7-epi-␣-selinene (10). Tentative designations are labeled with an asterisk. Structures of major compounds shown from left to right include valencene,
⌭,⌭-␣-farnesene, and 7-epi-␣-selinene.
and VI, but decreased significantly at bloom and fruit set (Fig. Red O for light microscopy. The images presented in Fig. 3D and
3B). Apparently, the sesquiterpene volatiles were released E show dense clusters of lipid bodies (seen as globular red dots)
quickly after flowers and anthers opened. In contrast, aliphatic inside the pollen grains.
compounds and diterpenes (one diterpene, geranyllinalool, com-
posed 70%–95% of the diterpenes identified) remained high at Valencene Synthase Produces Sequiterpene Volatiles in Grapevine
bloom and were still present in anthers at fruit set, presumably Anthers. In previous work (18), we functionally characterized a
due to their lower volatility and/or continued biosynthesis of cDNA encoding (⫹)-valencene synthase (VvValGw) responsible
these compounds. for producing (⫹)-valencene and (⫺)-7-epi-␣-selinene and 5
minor sesquiterpenes from V. vinifera L. cv. Gewürztraminer.
Flower Volatiles in Anthers Are Associated With Pollen Grains and Because (⫹)-valencene and (⫺)-7-epi-␣-selinene are 2 of the 3
Pollenkitt. To test for the presence of volatiles in the surface most abundant sesquiterpenes in the volatile emissions of Cab-
pollenkitt or within the pollen grains, we separated pollen from ernet Sauvignon flowers (Table S1), we cloned and functionally
anthers at stages V and VI and extracted volatiles according to characterized VvValCS cDNA from Cabernet Sauvignon flow-
the method described by Dobson et al. (17). This method relies ers. The full-length VvValCS cDNA contains an ORF of 1,671 nt
on rapid pentane extraction to produce compounds present in and encodes a protein of 556 aa. The VvValCS sequence is nearly
the pollenkitt and a longer extraction with chloroform/methanol identical (99% at the nt and aa levels) to VvValGw (Fig. S3).
for compounds present inside the pollen grain. At stage V, most His-tagged VvValCS was expressed in Escherichia coli, and the
volatiles were found in the chloroform/methanol fraction, indi- purified sesquiterpene synthase enzyme activity showed the
cating that these compounds may be produced inside the pollen same product profile (Fig. S2B), dominated by (⫹)-valencene
grain (Fig. 3C and Fig. S2 A). Analysis of stage VI pollen grains and (⫺)-7-epi-␣-selinene, as VvValGw with farnesyl diphosphate
revealed volatiles both inside and outside of the pollen grain. (FPP) as the substrate. The sesquiterpene products of VvValCS
These compounds may be transported to the pollenkitt before have similar retention time and mass fragmentation patterns as
Downloaded by guest on November 4, 2021
anthesis to facilitate volatile release. To visualize lipids that may those found in the flower volatile emissions and anther extracts,
serve for prebloom sequestration of lipophilic volatiles inside the except for ⌭,⌭-␣-farnesene and an aliphatic methylketone,
pollen grain, anthers were cryosectioned and stained with Oil 2-tridecanone (Fig. 3F).
Martin et al. PNAS 兩 April 28, 2009 兩 vol. 106 兩 no. 17 兩 7247molecular weight when tested in immunoblotting with recombinant
A 18
VvValGw and VvValCS protein (Fig. S4A). Anti-VvVal did not
Relative Expression
give signals in Western blotting when tested against 11 other
12 grapevine recombinant terpene synthases (data not shown). VvVal
protein was detected at prebloom stages V and VI and, to a slightly
lesser extent, at bloom (Fig. 4B). VvVal was not detected at any of
6
the other flower or berry stages or in roots, seeds, or leaves (Fig.
S4 B and C). The results from VvVal protein measurements over
n.d. the developmental time course are consistent with the presence of
0
I-III IV V VI Bloom the sesquiterpene products from VvVal enzyme activity in stage V
Stage of Flower Development and stage VI flowers (Fig. 3 B and C), which is preceded by the peak
of VvVal transcripts at stage IV (Fig. 4A).
B Flower Berry To localize VvVal during flower development, we dissected
III IV V VI B FS PS flowers into anthers, pistils, and sepals during stages V and VI
70
αVal 55
and bloom. Immunoblot analysis demonstrated the presence of
VvVal in the protein isolated from anthers, but not in that
55
αActin isolated from pistils or caps (Fig. 4C).
40
VvVal Is Localized Within Pollen Grains. Based on the detection of
C Anther Pistil Cap VvVal in anthers at stages V and VI and bloom (Fig. 4C), we
V VI B V VI B V VI B investigated VvVal localization at the level of specific tissue or
70
αVal cell type. For the immunohistochemical analyses, we used VvVal
55
antibody in combination with Alexa-488 secondary antibody and
55
αActin fluorescent microscopy on resin-embedded flower samples at 6
40 developmental stages (II–VI and bloom) to visualize VvVal
within flower tissues. Sections of the resin-embedded samples
Fig. 4. Stage-specific expression of VvValCS gene and VvValCS protein. (A)
Quantitative RT-PCR analysis of VvValCS mRNA during flower development.
were labeled with either VvVal antibody or preimmune serum.
VvValCS transcript abundance is shown at stages I–VI relative to transcript Floral tissues had a considerable amount of autofluorescence in
abundance at bloom. Each bar represents measurements from 3 technical both channels. Autofluorescence was particularly strong in pol-
replicates for each of 2 biological replicates ⫾ SEM. GAPDH was used as an len grains, as reported previously (19).
endogenous control. ⌬Ct for stages I–III was ⬍2 cycles different from no Consistent with the immunoblot analyses, VvVal was not de-
template controls ⫽ no expression. (B) Protein expression of VvValCS in flower tected by immunohistochemical in situ analysis at the early pre-
(stages I—VI and bloom) and berry stages (fs, fruit set; ps, pea size). SDS/PAGE bloom stages II–IV (data not shown), whereas sections from stages
gels were loaded with protein from ⬇3 mg of tissue. Actin was measured as a V and VI showed discrete areas of punctuate fluorescence signals
loading control. (C) Protein expression of VvValCS in anthers, pistils, and caps
in the presence of VvVal antibody localized to the inside of pollen
at stages V and VI and bloom. Gels were loaded with protein for each flower
organ pooled from 15 flowers for each stage of development. Actin was
grains (Fig. 5 B and D and Fig. S5 A and C). The VvVal-specific
measured as a loading control. fluorescence signal was localized primarily around spherical bodies
within the pollen grain (Fig. 5 D and Fig. S5 C). No such signal was
found in sections treated with preimmune serum instead of VvVal
To further substantiate the localization of sequiterpene bio- antibody (Fig. 5 C and E and Fig. S5 B and D) and in no–primary
synthesis, we measured sesquiterpene synthase enzyme activity antibody controls. Some additional fluorescence signal localized
in total protein isolated from anthers. These assays revealed 2 VvVal to the intine/exine region of several pollen grains at stages
sesquiterpene synthase products, valencene and 7-epi- ␣ - V and VI (Fig. 5 B and D and Fig. S5 C). No substantial signal was
selinene, produced at a rate of 0.184 ⫾ 0.002 and 0.186 ⫾ 0.009 seen between the pollen grains (inside the locule of the anther), and
pmol sesquiterpene/mg protein/h, respectively. Neither of these little label was detected on the outside of the pollen grains,
compounds (nor any other sesquiterpene) was detected in the suggesting that VvVal protein is localized primarily to the inside of
no-substrate or no-enzyme control assays. Sesquiterpene syn- the pollen grains, not to disintegrating tapetum cells or the pollen-
thase enzyme activity was not detected in protein extracts kitt. In addition, no signal was seen in the cells composing the
obtained from any other floral organ. These results support a anthers and filaments.
When visualized under high magnification (63⫻), sections
role for VvValCS in the formation of volatiles in anthers. We did
treated with anti-VvVal revealed clusters of punctate signals
not detect ⌭,⌭-␣-farnesene (the third major sesquiterpene vol-
around the outside of spherical bodies. Whereas pollen grains
atile in Cabernet Sauvignon flowers) as a product in these
have a number of distinct organelles and subcellular structures
sesquiterpene synthase assays. such as lipid bodies, plastids, and vacuoles (20), these spherical
bodies likely are lipid vesicles similar to those identified by light
VvValCS Transcripts Are Most Abundant at Prebloom, Whereas VvVal
microscopy with Oil Red O staining (Fig. 3 D and E). This
Protein Accumulates at Prebloom and at Bloom. To understand how the observation is supported by visualization of anti-VvVal immu-
synthesis of sesquiterpene volatiles is controlled at the transcript nolabeling using TEM (Fig. 6 A–F and Fig. S6 A–F). TEM
level in grapevine flowers, we isolated RNA from inflorescences at revealed stage VI flowers with substantial amounts of anti-
all prebloom stages I–VI and at bloom and used quantitative VvVal label localized primarily at the outer edges of individual
real-time PCR (qRT-PCR) to measure VvValCS transcript abun- or merging lipid vesicles. No label was seen in the control, and
dance. Whereas VvValCS transcripts were below the detection limit very little label was seen in other portions of the pollen grain.
at stages I–III (Ct values ⬍ -2 no template control Ct), transcript
abundance dramatically increased at stage IV (Fig. 4A), followed by Discussion
a decrease at stages V and VI and at bloom. Using several lines of evidence, including results from metabolite
To complement transcript analysis with protein detection, we
Downloaded by guest on November 4, 2021
analysis of headspace volatile collections and anther and pollen
used a polyclonal antibody raised against a 15-aa synthetic peptide extracts, VvValCS enzyme activity assays, and VvValCS protein
(Fig. S3) from VvVal. The antibody gave single bands of the correct localization, we have shown that anthers—more specifically,
7248 兩 www.pnas.org兾cgi兾doi兾10.1073兾pnas.0901387106 Martin et al.A c Pollen grain preimmune
A B
a n
v
f
p preimmune αVvVal
Alexa488 Hoechst Merged C D
αval
n
B
v v
E αVvVal F αVvVal
C preimmune
v
v
αval
D
Fig. 6. TEM immunogold localization of VvVal within pollen grains. (A) Pollen
grain with a single nucleus (n). (Scale bar ⫽ 2 m.) (B–F) Pollen grain sections with
lipid vesicles (v). Arrows indicate fusions of vesicles. Immunogold labeling of
preimmune ␣-VvVal primary is highlighted in red after imaging; the original image is pro-
E vided for reference as a supplementary figure. Most of the label is present on the
outer edges of these lipid vesicles. No label is seen in the preimmune control.
[Scale bar ⫽ 500 nm for (B), (C), (E), and (F) and 100 nm for (D).].
Fig. 5. Immunofluorescence localization of VvValCS protein to the pollen primarily from VvValCS in the developing microspores. The
grains of stage V flowers. (A) Longitudinal section of a grape flower (f) stained biosynthesis of terpene volatiles within anthers and pollen grains
with toluidine blue. The box highlights the portion of the anther (a) imaged in before bloom presents a substantially different biological system
(B–M). The pistil (p) and the cap (c) are indicated in white. (Scale bar ⫽ 200 m.)
of floral scent formation than those described from species
(B–E) The first column of images shows fluorescence from VvVal primary antibody
with Alexa-488 secondary, the second column shows the same section with 332
studied previously, in which floral scent biosynthesis occurs
Hoechst staining of nuclei, and the third column shows the 2 channels overlaid. during the blooming process and is been attributed to other
PLANT BIOLOGY
VvVal is seen in the images with discrete green clusters. Diffuse autofluoresence flower tissues but not the male flower parts (12).
is present for the blue and green channels for all treatments. (B and C) Images of
a section of an anther locule containing pollen grains. VvVal is localized primary Materials and Methods
inside the stage V pollen grains. Some label is found on the edges of the pollen Plant Material. V. vinifera L. cv. Cabernet Sauvignon canes (clone 15 grafted on
grains. Little label is found on the outside of the pollen grains or in other parts of rootstock 101–14) containing 6 nodes were harvested from Bull Pine Estate
the anther locule. (Scale bar ⫽ 50 m.) (D and E) High-magnification (63⫻) images Vineyards (Vincor), Osoyoos, BC in January 2007 and 2008 and kept at 4 °C
of representative pollen grains from stages V. Most of the label is present within before rooting. Plants were grown as described by Mullins and Rajasekaran
the pollen grain, although some label is seen on the inner edges of the pollen (24) with some modifications, as detailed in SI Materials and Methods.
grains as well. (Scale bar ⫽ 10 m.)
Volatile Collection. An automated volatile collection system (Analytical Re-
search Systems) was used as described previously (25). Details are given in SI
pollen grains—are the site of sesquiterpene volatile synthesis in Materials and Methods.
grapevine flowers. The presence of these volatiles precedes
flower opening. Gene expression analysis found that the highest Flower and Pollen Extractions. For each stage (V to bloom), 10 flowers were
transcript abundance for VvValCS is from stage IV flowers, separated into anthers, caps, and pistils/pedicels, and the individual flower
preceding the peak of VvValCS protein and the presence of parts were immediately submerged in 0.5 mL of pentane containing 2.0 g/mL
volatile compounds. Whereas VvValCS accounts for 2 of the 3 of isobutylbenzene as an internal standard. Volatiles were directly extracted
major grapevine f lower volatiles, the enzyme for ⌭,⌭-␣- from pollen grains using the method specified by Dobson et al. (17) with
farnesene synthesis remains to be identified in the numerous modifications, as described in SI Materials and Methods.
terpene synthases in the grapevine genome (21, 22).
Immunofluorescence analysis throughout flower develop- GCMS Analysis. GCMS analysis was performed as described previously (26).
Details are provided in SI Materials and Methods.
ment showed VvValCS protein clearly localized within pollen
grains, and transmission electron microscopy (TEM) localized
VvValCS cDNA Cloning and Recombinant Protein Enzyme Assays. RNA was
VvValCS with the outer edge of lipid vesicles, which are abun-
extracted from flowers, and cDNA was made as described by Reid et al. (27).
dant in the maturing microspore but no longer seen at anthesis. VvValCS cDNA was isolated by PCR using primers designed against the TIGR
Whereas tapetum cells have been attributed to the production of contig TC38950. VvValCS and the previously described VvValGw (18) were
lipids, phenylpropanoids, proteins, carbohydrates, and carote- cloned into pET28b(⫹) (Novagen) and expressed as C-terminal His-tag fusion
Downloaded by guest on November 4, 2021
noids, which eventually compose the pollenkitt or pollen coat (4, proteins in E. coli C41 cells containing the pRARE 2 plasmid (28). Single-vial
5, 23), our results do not support a role of tapetum cells in the assays were used for functional characterization, as described previously (28,
ontogenesis of sesquiterpene volatiles, which seems to arise 29). Additional details are provided in SI Materials and Methods.
Martin et al. PNAS 兩 April 28, 2009 兩 vol. 106 兩 no. 17 兩 7249Enzyme Assays With Protein from Anthers. Anthers were dissected from 100 and 332 Hoechst nuclei staining was imaged with an AMCA filter (#31000;
stage VI flowers, frozen in liquid nitrogen, and ground with sea sand in a Chroma Technology). All images were analyzed using AxioVision 4.7 (Carl
mortar and pestle for 5 min. Then 5 mL of extraction buffer [50 mM Tris (pH Zeiss).
7.5), 10% polyvinylpolypyrrolidone (wt/vol), 1% polyvinylpyrrolidone-40 (wt/
vol), 2 mM DTT, 0.2% activated charcoal, 10% (wt/vol) glycerol, and 1 mM TEM. Resin-embedded sections that gave signals for VvVal localization were
PMSF] was added. Extracts were sonicated using a Branson digital sonifier at resectioned using an ultra-diamond knife (50 nm thick) and then adhered to
15% power for 1 min on ice, and then ground with a mortar and pestle for formvar-coated nickel grids. Immunolabeling was done as described above
another 5 min. The extract containing soluble protein was centrifuged at but using 5% BSA instead of skim milk, and all TBST buffers were filtered
3500 ⫻ g for 30 min at 4 °C. Supernatant was further cleared at 21,000 ⫻ g for through a 0.22-m filter before use. Secondary labeling was done with 10-nm
30 min at 4 °C and desalted using PD MiniTrap G-10 columns (GE Healthcare gold goat anti-rabbit (British Biocell). TEM imaging was performed using a
Life Sciences). Enzyme assays were performed as described for recombinant Hitachi H7600 transmission electron microscope operating at 80 –100 kV.
enzyme assays, but with 0.25 mL of desalted protein extract (0.65 mg protein/
mL) used for each assay. Controls included assays without substrate or without
Light Microscopy. Stage VI anthers were embedded and frozen in OCT medium
protein.
before being cryosectioned at a thickness of 25 m. Sections were melted onto
a slide, fixed for 10 min in 4% paraformaldehyde, and stained with Oil Red O
Protein Extraction for Western Blot Analysis. Protein from individual flowers
(0.1 g of Oil Red O in 60% isopropanol) for 15 min. After a rinse with 60%
was extracted by resuspending powdered frozen plant tissue in loading
isopropanol, the sections were covered with mounting medium and visualized
buffer. For floral organs, total protein was extracted as described by Holmes-
by light microscopy.
Davis et al. (30). These procedures are detailed in SI Materials and Methods.
qRT-PCR. Flowers at stages I–VI and bloom were used for RNA isolation, cDNA
Immunoblot Analysis. Immunoblot analyses followed standard procedures
synthesis, and comparative Ct RT-PCR analysis as described by Reid et al. (27).
using polyclonal VvVal antibodies made against a synthetic peptide
Details are provided in SI Materials and Methods.
(EDLKKEVKRKLTAAC) by Sigma-Genosys (Sigma-Aldrich). Details of the meth-
ods are provided in SI Materials and Methods.
ACKNOWLEDGMENTS. We thank Dr. Oliver Prange for helpful discussions and
microscopy assistance, the Alaa El-Husseini Laboratory for the generous use of
Immunohistochemistry and Fluorescence Microscopy. Flower tissues were fixed
their microscopes, and Vincor Canada for access to their vineyards for cane
and embedded as described previously (31), and immunolabeling using collection. This research was supported by grants from Genome Canada and
␣-VvVal was done following standard procedures, as described in detail in SI Genome British Columbia (to J.B. and S.T.L.) and the Natural Sciences and
Materials and Methods. Fluorescence microscopy was done using an AxioVert Engineering Research Council of Canada (postdoctoral fellowship to D.M.M.;
200M and an AxioImager Z1 fitted with an Apotome (Carl Zeiss). Alexa 488 was discovery grant and E.W.R. Steacie Memorial Fellowship to J.B.). J.B. is a
imaged using an EndowGFP bandpass filter (#41017; Chroma Technology), University of British Columbia Distinguished Scholar.
1. Dobson HEM, Bergstroem G (2000) The ecology and evolution of pollen odors. Plant 18. Lücker J, Bowen P, Bohlmann J (2004) Vitis vinifera terpenoid cyclases. Phytochemistry
Syst Evol 222:63– 87. 65:2649 –2659.
2. Pellmyr O, Thien LB (1986) Insect reproduction and floral fragrances. Taxon 35:76 – 85. 19. Zhang XY, et al. (2006) A shift of phloem unloading from symplasmic to apoplasmic
3. Ashman T-L, Cole DH, Bradburn M, Blaney B, Raguso RA (2005) The scent of a male. pathway is involved in developmental onset of ripening in grape berry. Plant Physiol
Ecology 86:2099 –2105. 142:220 –232.
4. Pacini E, Hesse M (2005) Pollenkitt: Its composition, forms and functions. Flora (Jena) 20. Pacini E (2000) From anther and pollen ripening to pollen presentation. Plant Syst Evol
200:399 – 415. 222:19 – 43.
5. Piffanelli P, Ross JHE, Murphy DJ (1998) Biogenesis and function of the lipidic structures 21. French-Italian Public Consortium for Grapevine Genome Characterization (2007) The
of pollen grains. Sex Plant Reprod 11:65– 80. grapevine genome sequence suggests ancestral hexaploidization in major angiosperm
6. May P (2000) From bud to berry, with special reference to inflorescence and bunch phyla. Nature 449:463– 467.
morphology in Vitis vinifera L. Aust J Grape Wine Res 6:82–98. 22. Velasco R, et al. (2007) A high-quality draft consensus sequence of the genome of a
7. Carmona MJ, Chaib J, Martinez-Zapater JM, Thomas MR (2008) A molecular genetic heterozygous grapevine variety. PLoS ONE 2:e3107.
perspective of reproductive development in grapevine. J Exp Bot 59:2579 –2596. 23. Wang A, et al. (2002) Male gametophyte development in bread wheat (Triticum
8. Kimura PH, Okamoto G, Hirano K (1998) The mode of pollination and stigma recep- aestivum L.). Plant J 30:613– 623.
tivity in Vitis coignetiae Pulliat. Am J Enol Viticult 49:1–5. 24. Mullins MG, Rajasekaran K (1981) Fruiting cuttings: Revised method for producing
9. Buchbauer G, Jirovetz L, Wasicky M, Herlitschka A (1994) Headspace analysis of Vitis test-plants of grapevine cultivars. Am J Enol Viticult 32:35– 40.
vinifera (Vitaceae) flowers. J Essent Oil Res 6:313–314. 25. Arimura G, Huber DP, Bohlmann J (2004) Forest tent caterpillars (Malacosoma disstria)
10. Buchbauer G, Jirovetz L, Wasicky M, Herlitschka A, Nikiforov A (1994) Aroma of white induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar
wine flowers. Z Lebensm Unters Forsch 199:1– 4 (in German). (Populus trichocarpa x deltoides). Plant J 37:603– 616.
11. Buchbauer G, Jirovetz L, Wasicky M, Nikiforov A (1995) Aroma from red wine flowers. 26. Martin DM, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis
Z Lebensm Unters Forsch 200:443– 446 (in German). and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol
12. Dudareva N, Pichersky E (2006) in Biology of Floral Scent, eds Dudareva N, Pichersky E 132:1586 –1599.
(CRC Press, Boca Raton, FL), pp. 55–78. 27. Reid KE, Olsson N, Schlosser J, Peng F, Lund ST (2006) An optimized grapevine RNA
13. Lee S, Chappell J (2008) Biochemical and genomic characterization of terpene syn- isolation procedure and statistical determination of reference genes for real-time
thases in Magnolia grandiflora. Plant Physiol 147:1017–1033. RT-PCR during berry development. BMC Plant Biol 6:27.
14. Mandaokar A, Kumar VD, Amway M, Browse J (2003) Microarray and differential 28. Keeling CI, Weisshaar S, Lin RP, Bohlmann J (2008) Functional plasticity of paralogous
display identify genes involved in jasmonate-dependent anther development. Plant diterpene synthases involved in conifer defense. Proc Natl Acad Sci USA 105:1085–1090.
Mol Biol 52:775–786. 29. O’Maille PE, Chappell J, Noel JP (2004) A single-vial analytical and quantitative gas
15. Staudt G (1999) Opening of flowers and time of anthesis in grapevines, Vitis vinifera chromatography–mass spectrometry assay for terpene synthases. Anal Biochem
L. Vitis 38:15–20. 335:210 –217.
16. Gribaudo I, Gambino G, Vallania R (2004) Somatic embryogenesis from grapevine 30. Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S (2005) Proteome
anthers. Am J Enol Viticult 55:427– 430. mapping of mature pollen of Arabidopsis thaliana. Proteomics 5:4864 – 4884.
17. Dobson HEM, Bergstroem G, Groth I (1990) Differences in fragrance chemistry between 31. Hudgins JW, Ralph SG, Franceschi VR, Bohlmann J (2006) Ethylene in induced conifer
flower parts of Rosa rugosa Thunb. (Rosaceae). Isr J Bot 39:143–156. defense. Planta 224:865– 877.
Downloaded by guest on November 4, 2021
7250 兩 www.pnas.org兾cgi兾doi兾10.1073兾pnas.0901387106 Martin et al.You can also read