The glycophosphatidylinositol anchor affects the conformation of Thy-1 protein
←
→
Page content transcription
If your browser does not render page correctly, please read the page content below
Journal of Cell Science 108, 487-497 (1995) 487
Printed in Great Britain © The Company of Biologists Limited 1995
The glycophosphatidylinositol anchor affects the conformation of Thy-1
protein
E. Barboni1, B. Pliego Rivero1, A. J. T. George2, S. R. Martin3, D. V. Renouf4, E. F. Hounsell4, P. C. Barber5
and R. J. Morris1,*
1Laboratory of Neurobiology, National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK
2Department of Immunology, Royal Postgraduate Medical School, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK
3Laboratory of Physical Biochemistry, National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK
4Glycoprotein Structure/Function Group, Department of Biochemistry and Molecular Biology, University College London, Gower
Street, London WC1E 6BT, UK
5Department of Pathology, University of Birmingham Medical School, Birmingham B15 2TT, UK
*Author for correspondence
B.P.R. and E.B. contributed equally to this paper
SUMMARY
Thy-1 has the structure of a single variable-type delipidation induces a conformational change in the Thy-1
immunoglobulin domain anchored to the external face of protein that is sufficiently strong to dissociate bound
the plasma membrane via a glycophosphatidylinositol antibody. This conformational change can be demon-
moiety. When the lipid is removed from this anchor by strated directly by the circular dichroism spectrum of
either phospholipase C or D, the reactivity of the delipi- human Thy-1 that detects changes in the environment of
dated Thy-1 for a range of antibodies, including those Tyr residues located near the antigenic epitopes. Molecular
known to be determined by amino acid residues, is dynamics studies suggest that, on delipidation, a confor-
impaired. We have investigated in detail the effect of delip- mational change occurs in the glycan chain that affects the
idation on the reaction with the OX7 monoclonal antibody, protein in the region of the antigenic epitopes. This study
determined by the allelic variant residue Arg 89. Analysis thus demonstrates that the glycophosphatidylinositol
of the kinetics of OX7 binding shows that delipidation anchor strongly influences the conformation of Thy-1
affects primarily the dissociation of antibody, increasing protein by a mechanism that could occur generally with
the dissociation rate constant kdiss from 0.27×10−3 s−1 to membrane proteins of this class.
2.39×10−3 s−1. Addition of phospholipase to preformed
antibody-antigen complex causes an immediate change Key words: antibody kinetics, delipidation, phospholipase, protein
from the slow to the faster dissociation rate, implying that modelling
INTRODUCTION anchor. The sequence of its GPI group (Homans et al., 1988),
its three carbohydrate chains (Parekh et al., 1987; Williams et
The glycophosphatidylinositol (GPI) group that anchors al., 1993) and its polypeptide chain of 111 amino acids
certain glycoproteins and proteoglycans to the cell surface can (Williams and Gagnon, 1982), which are folded in the β-
actively influence the function of the macromolecule that it pleated sheet of an immunoglobulin-type (Ig) variable domain,
tethers (Hooper, 1992). In certain cases this influence may be are known. Antibodies have been raised that detect an allelic
indirect, the GPI group serving to direct the protein to partic- variation in mouse Thy-1 at residue 89 (Williams and Gagnon,
ular functional microdomains on the surface where it interacts 1982). In some mouse (and all rat) strains, this residue is Arg,
with transmembrane signalling molecules concentrated there and the epitope so specified is called Thy-1.1; in most mouse
(Brown, 1993; Lisanti et al., 1994; Mayor et al., 1994; Tiveron strains this residue is Gln, specifying the Thy-1.2 epitope.
et al., 1994). There are other cases where the GPI anchor Residue 89 is at the top of the F strand of the β-pleated sheet,
appears to directly influence function, such as in the binding lying almost on the other side of the molecule from Cys 112,
by purified lymphocyte function associated antigen 3 (LFA 3, to which the GPI group is attached (Williams, 1989; see also
also known as CD58) of its ligand, CD2 (Tozeren et al., 1992). Fig. 9). It has been suggested, from molecular modelling
This functional interdependence of the GPI and polypeptide studies, that the glycan chain of the GPI anchor fits into a
domains raises the question as to whether there is any struc- lectin-type binding site on the adjacent surface of the protein,
tural interaction between these two regions. so that the bulk of the GPI anchor folds inside the Thy-1
Thy-1 is a particularly convenient protein in which to inves- protein (Rademacher et al., 1991).
tigate possible interactions between the protein and the GPI Here we report that delipidation of the GPI anchor of Thy-1,488 E. Barboni and others
by either phospholipase C or D (Fig. 1), changes the conforma- from ascitic fluid using Protein A affinity chromatography) of the
tion of the Thy-1 protein, as detected by antibody binding and OX7 mouse hybridoma clone (Mason and Williams, 1980), or alter-
circular dichroism. We further show that a substantial confor- natively with antibodies of the aPG22 clone (Greenspan and O’Brien,
mational shift in this region of Thy-1, brought about by delipi- 1989; Matthew et al., 1985). Antibodies were biotinylated with NHS-
LC-Biotin (Pierce) according to the manufacturer’s instructions. The
dation, is predicted by molecular modelling that further
Thy-1.2 epitope was detected using rat IgG antibodies of either the
suggests it is the relaxation of conformational contraints on the H129-93 (Pont et al., 1985) or 30H12 (Ledbetter and Herzenberg,
glycan anchor that in turn alters the protein conformation. 1979) clones. Mouse Thy-1 was also detected using rat monoclonal
antibodies to two other epitopes on Thy-1: H140-150 (against the ‘B’
epitope), and H154-177 (against the ‘C’ epitope) (Pont et al., 1985).
MATERIALS AND METHODS Human Thy-1 was detected by antibodies of the B7 clone (Miyata et
al., 1990). Rabbit anti-rat Thy-1 F(ab′)2 antibodies were as described
Antibodies used (Tiveron et al., 1994). Biotinylated donkey anti-rat IgG and anti-
The Thy-1.1 epitope was detected using IgG antibodies (prepared mouse IgG (Dako Ltd), biotinylated donkey anti-rabbit F(ab′)2 and
alkaline phosphatase-conjugated streptavidin (both from Amersham
International plc) were used to detect the primary antibodies.
Enzymes and digestion conditions
Thy-1 Phosphatidylinositol-specific phospholipase C, EC 3.1.4.10 (PI-PLC)
from Bacillus cereus, glycosyl-phosphatidylinositol-specific phos-
pholipase D, EC3.1.4.50 (PI-PLD) from bovine serum, and peptide-
N-glycosidase F, EC 3.2.2.18 (PNG-F), were from Boehringer
Mannheim. Bacillus thuringiensis PI-PLC was from Peninsula Labo-
NH ratories, or the recombinant enzyme from Oxford GlycoSystems.
Et For deglycosylation, Thy-1 (10 µg) was precipitated with 80%
acetone on solid CO2, then pelleted by centrifugation (11,750 g in a
P microfuge for 5 minutes at room temperature); the acetone was
removed by aspiration and vaporisation in an air stream, the protein
resuspended in 10 µl of 25 mM Tris-HCl, pH 6.8, with 0.5% SDS and
then heated at 100°C for 1 minute. On re-equilibrating at room tem-
perature, PNG-F (final concentration 2 units/ml) was added in 10 µl
of 100 mM Tris-HCl, pH 8.6/0.1% Triton X-100, 0.1% NP-40, 1 mM
phenylmethylsulphonyl fluoride (added immediately before use from
a 100 mM stock solution in dry ethanol) and 1 µg/ml 1,10-phenan-
throline. After 1 hour at 37°C the reaction was stopped by adding an
equal volume of non-reducing SDS-PAGE sample buffer (Laemmli,
1970).
Delipidation with PI-PLC was in 70 mM triethanolamine-Cl, pH
8.2/0.16% sodium deoxycholate; with PI-PLD, in 80 mM Tris-HCl
pH 6.7/4% ethanolamine/0.5 mM CaCl2. The precise conditions are
NH 2 EtP given in the text. Chemical delipidation was accomplished by mild
hydrolysis with 1 M hydroxylamine, pH 10.0, at 20°C for 12 hours
(Toutant et al., 1989).
Thy-1 purification
Rodent Thy-1 was purified from 28 day old rat (AS strain) or mouse
(A/Thy×1.1 strain, the congenic Thy-1 partner to the A/J strain) by
passage of a deoxycholate extract of brain membranes (post-nuclear
pellet (100,000 g for 1 hour) solubilised in 5% sodium deoxycholate
in 70 mM triethanolamine-Cl, pH 8.2) down an OX7 immunoaffinity
column (20 mg of OX7 IgG coupled to 10 ml of Pierce AminoLink);
bound antigen was eluted with 3 M KSCN in triethanolamine buffer
PI-PLD with 0.25% deoxycholate/0.02% NaN3. This was dialysed against
P 0.25% deoxycholate/triethanolamine/NaN3 and stored at 4°C. Human
PI-PLC Thy-1 was prepared from a sample of lateral temporal lobe removed
in the course of surgery; deoxycholate-solubilised membranes
prepared from it were passed down a B7 immunoaffinity column,
followed by gel filtration (below) to obtain the monomeric fraction.
This was available in much smaller quantities (The GPI anchor affects Thy-1 protein conformation 489
blots using nitrocellulose filters and antibodies as described below for (1:100 dilution), washed, incubated in streptavidin-alkaline phos-
immunoblots. The column was calibrated, in both detergent and phatase, and washed as before. Enzymatic activity was developed
detergent-free solution, with the following markers (with their using 0.5 mg/ml p-nitrophenyl phosphate in 100 mM Tris-HCl, pH
molecular mass): bovine cytochrome c (12.3 kDa); bovine chy- 9.4/100 mM NaCl/50 mM MgCl2; plates were read at 412 nm in a
motrypsinogen A (25.7 kDa); ovalbumin (43.5 kDa) and bovine TiterTek Multiskan MC photometer.
catalase (232 kDa). Blue Dextran was used to define the void volume
(4.75 ml). Kinetic analysis of antibody binding
Eluted fractions were analysed by SDS-PAGE (Laemmli, 1970) Kinetic analysis of antibody binding was performed using the IAsys
using non-reducing conditions. The following proteins were used as optical evanescent wave biosensor (Fisons Applied Sensor Technol-
standards: bovine cytochrome c, chymotrypsinogen A and ovalbumin ogy, Cambridge), with the sample stirred at 4,125 rpm. OX7 IgG (20
as above; bovine α-lactalbumin (14.2 kDa), soybean trypsin inhibitor µg/ml in 25 mM sodium acetate buffer, pH 4.5) was coupled via ε-
(21 kDa), and bovine carbonic anhydrase (29 kDa). Gels were stained amino groups to the carboxymethylated dextran layer (activated with
with Coomassie Blue R-250 or with silver (Gooderham, 1984); or the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccin-
protein transferred to nitrocellulose membrane and immunoblotted. imide) of the cuvette following the manufacturer’s instructions. The
The filter was blocked by incubation for 1 hour in 10% rehydrated cell was thermally equilibrated at 25°C in 200 µl of PBS plus 0.02%
defatted milk powder, and incubated for 30-60 minutes at room tem- Tween-20. Readings (5 averaged readings taken 0.2 second apart)
perature with biotinylated OX7 antibody (0.1 µg/ml in PBS with 1% were taken every 2 seconds. Thy-1 (2 µl at 0.1-12.5 µM) was added
milk powder), followed, after 3 washes over 15 minutes, with
and its binding followed; the cell was washed rapidly twice with PBS-
PBS/0.1% bovine serum albumin, with streptavidin-alkaline phos-
Tween, and its dissociation then followed. The cell was regenerated
phatase (10 ng/ml in 1% bovine serum albumin/PBS), or with rabbit
by removing bound Thy-1 with 20 mM HCl in 2× 1 minute washes,
anti-Thy-1 F(ab′)2 antibodies (1 µg/ml in 1% milk/PBS) followed by
then re-equilibrating with PBS-Tween. Similar results were obtained
biotin-labelled anti-rabbit antibodies (0.1 µg/ml in PBS/milk) and
streptavidin-alkaline phosphatase as above. The filter was washed using 0.25% deoxycholate/triethanolamine buffer instead of
with 150 mM NaCl/10 mM Tris-HCl, pH 7.5, then with 100 mM Tris- PBS/Tween.
HCl, pH 9.4/100 mM NaCl/50 mM MgCl2 (the latter added from 0.5 Data were analysed with the FASTfit programme (Fisons Applied
M stock immediately before use) and then incubated in the dark at Sensor Technology) (George et al., 1995). The association rate
room temperature in a sealed plastic bag from which the air was constant (kass) was determined by analysing the initial 70% of the
excluded, in the above buffer plus (per ml) 1.2 µl of 50 mg/ml 5- binding of varying concentrations of soluble Thy-1 (the monomeric
bromo-4-chloro-3-indolyl-phosphate and 5 µl of 75 mg/ml 4-nitro pool obtained from gel filtration in 0.25% deoxycholate/tri-
blue tetrazolium chloride, both in dimethylformamide. The reaction ethanolamine) to OX7 immobilised on the cell. FASTfit uses an
was stopped by washing with 10 mM EDTA, pH 7.5. iterative curve fitting procedure to fit the data to the equation:
Non-denaturing electrophoresis in deoxycholate was adapted from Rt = R0 + E(1−e−kobst) ,
published methods (Almqvist and Carlsson, 1988; Toutant et al.,
1989). Samples (20 µl) were electrophoresed at 10 V/cm for 3.5 hours where Rt is the response, measured in arcseconds, at time t (seconds),
towards the cathode (to identify delipidated forms) or anode (to and R0 is the initial response; E is the extent of change of response;
identify lipidated forms that bind deoxycholate) into 8% polyacry- and kobs is the observed rate constant.
lamide gels in 50 mM Tris/glycine, pH 8.7 with 0.5% Triton X-100 kobs is related to kass by the equation: kobs = kass[Thy-1] + kdiss. Thus
and 0.25% sodium deoxycholate. Protein was transferred to a nitro- a plot of kobs against Thy-1 concentration gives a straight line whose
cellulose filter by drying the gel against the filter (Bio-Rad gel dryer, slope is kass and intercept kdiss.
2 hours, 50°C); this was then blocked with milk and processed for The dissociation rate constants were calculated directly from the
Thy-1 immunoreaction as above. initial 10% of the dissociation reaction, by fitting the dissociation
Triton X-114 phase separations were done essentially as described curve to the equation:
by Bordier (1981). A 10% stock detergent solution in water was added Rt = Ee−kdisst .
to Thy-1 samples to give a 1% (v/v) Triton X-114 solution. Aliquots
The data points taken (70-200 for each curve) fitted the theoretical
(300 µl) in 400 µl microfuge tubes were shaken continuously for 30
curve for a single binding site to within 1 arcsecond, compared to a
minutes each, first in a waterbath held at 0°C with melting ice, and
then in a waterbath at 30°C. Aqueous and detergent phases were maximal response on binding Thy-1 of approximately 130 arcsec-
separated by centrifugation (MSE microcentrifuge, 11,750 g, 30 onds. A two-site binding equation was also fitted to the data, but did
minutes, room temperature) and removed using a very fine pipette tip not improve the fit.
(Mµlti-Flex, Anachem); both were made up to 300 µl final volume
Circular dichroism spectrum
with 0.9% NaCl/10 mM Tris-HCl, pH 7.4/1% bovine serum albumin,
and the Thy-1 content of each assayed by radio immune assay (Morris Thy-1 (400 µl at 0.1 mg/ml (rat Thy-1) or 10 µg/ml (human Thy-1)
and Raisman, 1983). in 0.16% deoxycholate/70 mM triethanolamine-HCl, pH 8.2) was
placed in a cuvette (1 cm path length) in a Jasco J-600 spectropo-
ELISA analysis larimeter, equilibrated at 37°C, and the spectrum from 260-350 nm
The reactivity of normal and delipidated Thy-1 was analysed by taken every hour for 3 hours; the sample was left overnight in the
comparing their ability to inhibit binding of anti-Thy-1 antibodies to cuvette, and a final 24 hour time point taken. PI-PLC was added to a
native Thy-1 immobilised on the ELISA plate. The buffer used was final concentration of 2.6 units/ml. Recombinant B. thuringiensis
10 mM Tris-HCl, pH 7.4/150 mM NaCl/0.05% Tween-20. A fixed enzyme, with a specific activity of >1000 units/mg protein, was used
concentration of antibody (1-2 µg/ml) was absorbed (overnight at to ensure that a high rate of hydrolysis was achieved with minimal
4°C, or 2 hours at room temperature) with serial 1:2 dilutions of Thy- protein added. Spectra were taken at 0.25, 0.5, 1, 3 and 24 hours. In
1 (starting at 1 µg/ml). Aliquots (50 µl) were transferred to the wells addition, 2 µl aliquots of sample were removed immediately after the
of a microtitre plate to which native mouse Thy-1.1 had been pre- spectra were taken, and their binding to OX7 analysed in the IAsys
adsorbed (by incubating it with 1 µg/ml Thy-1 in 0.1 M Na2CO3 cuvette. Complete conversion of the Thy-1 to the fast dissociating,
buffer, pH 9.6, for 1 hour at room temperature). The microtitre plates delipidated form was achieved within 30 minutes. Enzyme at the con-
were incubated at 37°C for 30 minutes, washed three times with centration employed here had no detectable circular dichroism (CD)
buffer, incubated as before in biotinylated rabbit anti-mouse IgG spectrum.490 E. Barboni and others
Molecular dynamics of the detergent forms large micelles with itself that have a
The coordinates for Thy-1 modelled on the immunoglobulin fragment molecular mass of approximately 300 kDa (Kuchel et al.,
Fab New were kindly supplied by Professor R. Dwek and Dr Mark 1978). On removal of the hydrophobic lipid groups by delipi-
Wormald (Oxford Glycobiology Institute) (Rademacher et al., 1991). dation, the resulting small, soluble glycoprotein should migrate
Oligosaccharide chains and the glycan and lipid of the GPI anchor in gel filtration as a monomer of molecular mass 17.5 kDa
from the original rat thymocyte Thy-1 model (Perkins et al., 1988) (Kuchel et al., 1978). When chromatographed in the presence
were reconstructed according to the chains suggested by Parekh et al. of 0.25% deoxycholate, native Thy-1 eluted with a minor peak
(1987) and Homans et al. (1988) using Insight II (Biosym Technolo-
gies), set up as described by Renouf and Hounsell (1993); and to be
of aggregated material at the void volume of the column; the
discussed in detail elsewhere. The molecule was treated in each of majority eluted as two peaks migrating with molecular masses
three ways: (a) immersed in a monolayer of lipids with phospho- of 23 (major) and 17 (minor) kDa (Fig. 3a; the doublet seen in
ethanolamine headgroups (approximating to a micelle or the cell the HPLC elution profile has been often observed after SDS-
surface); (b) delipidated at the phosphoinositol bond (i.e. as though PAGE, and is assumed to reflect heterogeneity in glycosy-
by PI-PLD); and (c) delipidated with the glycan of the membrane lation; Williams, 1989). Fractions from 10.0 to 11.5 ml, con-
anchor completely removed. Each of the molecules was submitted to
energy minimisation by conjugate gradients to a maximum derivative
less than 0.05 kcal/Å, followed by molecular dynamics at 300 K. A
distance-dependent dielectric constant of 4r and Biosym force field
CVFF were used throughout.
RESULTS
Effect of deglycosylation and delipidation on the
Thy-1.1 epitope
The effect on Thy-1 antigenicity, determined by immunoblot-
ting, of removal of each of the three N-linked carbohydrate
chains (using PNG-F), is shown in Fig. 2a. Partial deglycosy-
lation by PNG-F of purified rat Thy-1 displayed a ladder of
Thy-1 forms, ranging from the fully deglycosylated protein
(14.5 kDa) to forms with one (22 kDa) or two (18 kDa) car-
bohydrate chains removed. All these forms were recognised by
the monoclonal OX7 antibody, as well as the polyclonal rabbit
antibodies, confirming that the reaction of each does not
involve the carbohydrate chains of Thy-1. PI-PLC treatment
(Fig. 2b), however, reduced reactivity with the polyclonal anti-
Thy-1 antibodies, and completely eliminated reactivity with
the monoclonal anti-Thy-1.1 antibody.
Gel filtration analysis
Thy-1 in deoxycholate solution is monomeric, but on removal
Fig. 3. Separation of native and delipidated Thy-1 forms by gel
filtration: (A) elution profile, showing absorbance at 280 nm, of Thy-
1 chromatographed on the Protein Pak 300 column in 0.25%
deoxycholate. The inset gels show immunoblots of the fractions I
(eluting at the void volume) and II, labelled with the polyclonal (P)
Fig. 2. Effect of deglycosylation and delipidation upon Thy-1 and OX7 monoclonal (M) antibodies; (B) elution profile of fraction
antigenicity: (a) immunoblot of samples following SDS-PAGE in a II, rechromatographed on the column equilibrated now in
15% acrylamide gel, of native Thy-1 (lanes 1,3 ) and Thy-1 partly triethanolamine buffer without deoxycholate; inset shows
digested with PNG-F to produce a ladder of deglycosylated forms immunoblot of major peak, labelled with the polyclonal and
(lanes 2,4), immunolabelled with rabbit anti-Thy-1 (lanes 1,2) and monoclonal antibodies as before; (C) fraction II, delipidated with PI-
OX7 anti-Thy-1.1 (lanes 3,4) antibodies; (b) immunoblot of similar PLC, rechromatographed in the absence of deoxycholate showing
samples of either native Thy-1 (lanes 1,3) or Thy-1 delipidated with (inset) the immunoreactivity for the polyclonal (P) and monoclonal
PI-PLC (lanes 2,4), immunolabelled with rabbit (lanes 1,2) or OX7 (M) antibodies of the pooled (11-12.25 ml) peaks; (D) elution profile
(lanes 3,4) antibodies. Values on the left are kDa. and immunoblot after PI-PLD delipidation of fraction II.The GPI anchor affects Thy-1 protein conformation 491
taining this doublet of monomeric Thy-1, were pooled and
rechromatographed down the column under different con-
ditions. When the column was equilibrated in deoxycholate as
before, all the Thy-1 was recovered in the two peaks of lower
molecular mass (not shown); when however the column was
equilibrated in triethanolamine buffer without detergent, the
major peak of Thy-1 eluted at the void volume (Fig. 3b). The
fully lipidated Thy-1 in these three peaks was reactive on
immunoblots with both OX7 anti-Thy-1.1 and rabbit anti-Thy-
1 antibodies (insets in Fig. 3a,b). However, when the
monomeric peak recovered after filtration in detergent (peak II
in Fig. 3a) was delipidated with either PI-PLC (Fig. 3c; 30-40
µg Thy-1 digested with 50 munits/ml B. cereus PI-PLC, 3
hours at 37°C) or PI-PLD (Fig. 3d; 30-40 µg Thy-1 with 25
units/ml enzyme as above), the Thy-1 migrated in detergent-
free buffer as a doublet (15 and 12 kDa) that reacted with the
rabbit but not the OX7 antibodies (insets in Fig. 3c,d).
The small fraction (a few per cent) of Thy-1 that eluted in
the void volume after phospholipase digestion (Fig. 3c,d) was
resistant even to a second digestion with the enyzme and was Fig. 4. Immunoreactivity of native and delipidated Thy-1 following
electrophoresis in deoxycholate: (a) Immunolabelling with rabbit
detectable by both the rabbit and OX7 antibodies. Following
anti-Thy-1 antibodies, and (b) with OX7 anti-Thy-1.1 antibodies, of
mild hydrolysis with hydroxylamine (to remove acylation of nitrocellulose membrane onto which had been blotted 8%
the inositol that protects from PI-PLC action; Toutant et al., polyacrylamide gels in 0.25% deoxycholate with: native Thy-1 (lane
1989), approximately 20% of this fraction became sensitive to 1), PI-PLD-treated Thy-1 (lane 2) and PI-PLC-treated Thy-1 (lane
PI-PLC with a concomitant loss of reactivity to OX7 antibodies 3); upper panel, migration towards cathode (0, origin; _, cathode);
(not shown). lower panel, migration towards anode (0, origin; +, anode).
Analysis of delipidation by binding of non-
denaturing detergents
hold Thy-1 together despite proteolytic cleavage. This would
Delipidation, in removing the detergent-binding lipid chains be evident with reduced samples (which do not show up on
from Thy-1, changes its electrophoretic behaviour in non-dena- silver staining). Fig. 5b shows Coomassie Blue staining of
turing ionic detergents, and its partitioning between the reduced samples after SDS-PAGE, and again no proteolytic
detergent and non-detergent phases created by temperature- fragments of Thy-1 are evident.
shift of an aqueous solution of Triton X-114. Both these assays
can be used to assess the effect of delipidation on antigenicity.
Fig. 4 shows an immunoblot after electrophoresis in 0.25% Generality of effect of delipidation upon Thy-1
deoxycholate at pH 8.7. Under these conditions, only Thy-1, antigenicity
completely delipidated with either PI-PLD (lane 2) or PI-PLC The loss of reactivity of delipidated rat Thy-1 for the OX7 anti-
(lane 3), migrated towards the cathode (upper panels), where Thy-1.1 antibodies was not restricted to antibodies of this clone
it was detected by the rabbit (Fig. 4a) but not OX7 (Fig. 4b) - the reaction of aPG22 anti-Thy-1.1 antibodies were similarly
antibodies. Native Thy-1, with its lipid chains binding the affected (not shown). A wider range of anti-Thy-1 antibodies
deoxycholate anion, moved towards the anode (lower panels) can be tested in the mouse. Fig. 6 shows competitive ELISA
where it was detected with both the rabbit and OX7 antibodies. assays, in which purified mouse Thy-1.1, with or without delip-
Delipidated Thy-1 partitions into the aqueous, rather than idation by PI-PLC, has been incubated with three monoclonal
detergent, phase of Triton X-114 (Almqvist and Carlsson, antibodies that detect spatially adjacent, but separate, epitopes
1988). Over 95% of delipidated Thy-1 could be detected by on Thy-1 (Pont et al., 1985); unreacted antibody has then been
radioimmunoassay in the aqueous phase using rabbit anti- measured by its binding to native mouse Thy-1.1 on the ELISA
bodies, but only trace amounts (1-5%) could be detected there plate. Fig. 6A shows the reaction of OX7 with the mouse Thy-
with OX7 antibodies (not shown). 1.1 epitope; Fig. 6B and C the reaction of H140-150 and H154-
177 with the B and C epitopes, respectively, on Thy-1 (Pont et
Does proteolysis accompany delipidation? al., 1985). For each of these, delipidation dramatically affects
Could proteolysis accompany delipidation and so affect the the reaction of the antibody. In addition to these studies with
Thy-1 epitopes? To test for this, samples (0.1 µg) of Thy-1 purified Thy-1, Thy-1 was removed from thymocytes and
were incubated (3 hours, 37°C) with increasing amounts of PI- transfected cell lines in culture by PI-PLC (Barboni et al.,
PLC, ranging from 0.5 munit/ml (sufficient to completely 1991; Tiveron et al., 1994); the Thy-1 recovered in the super-
delipidate this quantity of Thy-1) up to 50 munits/ml. The natant could be detected in immunoblots with the rabbit anti-
samples, without reduction, were analysed by SDS-PAGE bodies, but not the monoclonals tested (not shown). Finally,
followed by silver staining (Fig. 5a). At higher concentrations although the rabbit antibodies retained reactivity for delipi-
of enzymes, protein bands derived from the enzyme prepara- dated Thy-1, this was clearly reduced (e.g. Fig. 2b); in quan-
tion became visible (lanes 5, 6) but no evidence of proteolysis titative assays (ELISA and RIA) this reduction was found to
of Thy-1 was seen. It is possible that its two disulphide bonds be approximately 50% (not shown).492 E. Barboni and others
A
0.5
Absorbance at 412nm
0.0
B
0.5
Fig. 5. Lack of proteolysis during delipidation: (a) silver-stained
15% acrylamide gel on which 0.1 µg of non-reduced Thy-1 was
electrophoresed following treatment with 0, 0.5, 2.5, 5.0, 25 and 50 0.0
munits/ml PI-PLC (lanes 1-6); lane 7 contained no Thy-1 and 70 C
munits/ml of PI-PLC; (b) Coomassie Blue staining of a gel on which
reduced samples of 14 µg of Thy-1 were electrophoresed; lane 1,
native Thy-1; lane 2, Thy-1 incubated for 3 hours with 50 mU/ml PI-
0.5
PLC.
Kinetic analysis of the effect of delipidation upon
OX7 binding of rat Thy-1
To obtain a more precise view of the effect of delipidation
0.0
upon antibody binding to Thy-1, the kinetics of OX7 binding
512 128 32 8 2
were analysed using an optical evanescent wave biosensor
(employing the IAsys system). This allows the interaction of Reciprocal Dilution
molecules to be studied, in real time, by use of a resonant
Fig. 6. Delipidation affects epitopes of mouse Thy-1.1. ELISA
mirror device. Changes in the sensing layer, by altering the
analysis of antibody binding remaining after absorption of
properties of an evanescent wave, lead to alteration in the monoclonal antibodies with increasing dilutions of native (s) and
resonant waveguide mode that can be readily detected by mon- delipidated (d) Thy-1. The antibodies used were: (A) OX7,
itoring the resonance position (Buckle et al., 1993; Cush et al., recognising the Thy-1.1 (or A) epitope; (B) H140-150, recognising
1993). In these experiments the OX7 antibody was immo- the B epitope; and (C) H154-177, recognising the C epitope.
bilised onto the dextran hydrogel that extends 200-500 nm
from the sensing surface. Soluble, monomeric Thy-1 (gel
filtered in deoxycholate as above) was added and its binding The effect of delipidation upon dissociation was further
to the immobilised OX7 followed. investigated by assessing whether PI-PLC could influence the
Fig. 7A shows traces for the binding, and subsequent disso- dissociation of pre-formed OX7/Thy-1 complex. Native Thy-
ciation, of both native (upper trace) and delipidated (lower 1 was first bound to the antibody (Fig. 7B), then the buffer
trace) Thy-1 from immobilised OX7. The association reactions changed (at 1000 seconds) to initiate dissociation as before, but
of the two Thy-1 forms follow very similar kinetics. In the this time containing 2.6 units/ml of recombinant B. thuringien-
inset, the observed rate constants are plotted as a function of sis PI-PLC. The dissociation rate observed was the faster rate
initial Thy-1 concentration, the slopes giving values for kass of typical of delipidated Thy-1 (Fig. 7B, 1000-1160 seconds). The
5.98±1.05×104 M−1 s−1 (native Thy-1) and 8.25±0.05×104 buffer was then changed (at 1165 seconds) to PBS/Tween
M−1 s−1 (delipidated Thy-1). It is immediately obvious from without the lipase, and the dissociation reverted to the slower
the primary experimental data that the dissociation reaction rate of non-delipidated Thy-1.
proceeds considerably faster with delipidated, than with native,
Thy-1. The dissociation rate constant, kdiss, was Circular dichroism spectrum of the delipidation
0.27(±0.03)×10−3 s−1 for native Thy-1, and 2.39(±0.65)×10−3 reaction
s−1 for delipidated Thy-1, giving overall dissociation constants Circular dichroism was used to follow any changes in tertiary
(kd) of 4.5×10−9 M and 2.9×10−8 M for native and delipidated structure accompanying delipidation of Thy-1 (Fig. 8). The
Thy-1, respectively. The FASTfit software used to analyse the near UV CD spectrum of rat Thy-1 shows a strong signal
data allows the reaction kinetics to be modelled to single, or centred on 280 nm and significant long wavelength intensity.
dual, binding site reactions. The fit of the experimental data to Given the absence of Trp residues in Thy-1 (Williams, 1989),
the one-site model was excellent, within the low noise level of this feature must be due to one of the disulphide bonds in the
the system (see Materials and Methods), and was not improved protein (Strickland, 1974). The two Tyr residues in rat Thy-1
by using the two site model. The same dissociation rate was (conserved in human and mouse Thy-1; Williams, 1989) pre-
obtained whether it was measured on the initially dissociating sumably contribute to the signal at wavelengths shorter than
fraction, or (after fresh buffer changes to remove dissociated 290 nm. Sufficient PI-PLC was added to ensure delipidation
Thy-1) on fractions after more than 50% of the Thy-1 origi- was complete in less than 30 minutes, as monitored by con-
nally bound to the cuvette had dissociated. version to the rapidly dissociating form in the IAsys OX7The GPI anchor affects Thy-1 protein conformation 493
100 A B
80
Position (Arc secs)
60
2.5
40
2.0
k obs x 10 2
1.5
20 1.0
0.5
0
0 0.5 1.0 1.5 2.0
7
0 [Thy-1] x 10
200 250 300 350 400 450 500 550 600 650 600 800 1000 1200 1400
Time (secs)
Fig. 7. Association and dissociation of the Thy-1/OX7 reaction demonstrated by the IAsys biosensor. (A) Binding of normal (upper trace) and
delipidated (lower trace) Thy-1 to OX7 IgG immobilised on the surface of the cuvette. After establishing a linear baseline, buffer containing
Thy-1 (25 nM normal or 12.5 nM delipidated Thy-1; different concentrations being used here to ensure the two traces could be seen separately)
was added at 220 seconds and its association followed for 100 seconds, at which time the cuvette was washed with PBS/Tween (deflection in
trace at 320 seconds) to start dissociation, which was followed for 400 seconds. Inset, a plot of kobs (s−1) versus Thy-1 concentration (M) for
normal (d) and delipidated (s) Thy-1, the slope of which gives the forward reaction rate (kass). (B) Normal Thy-1 was bound to the OX7
antibodies (585-985 seconds); fresh buffer containing PI-PLC was then added (990-1160 seconds), which in turn was twice replaced (at 1160
and 1330 seconds) with PBS/Tween without the enzyme. The shift of 6 arcseconds on adding or removing the PI-PLC/PBS/Tween solution is
an effect of the buffer change, not antigen dissociation/association.
cuvette; no change in the near UV CD spectrum of rat Thy-1 PI-PLC addition)), which is most probably associated with a
was observed to accompany delipidation (Fig. 8). change in the environment of one or more Tyr residues.
Human Thy-1 has three Tyr residues not present in rat Thy-
1, at amino acid positions 32, 66 and 72, the first two of which Molecular modelling of the effect of delipidation
are located on loops (B-C and D-E) that are adjacent to, or upon Thy-1
within, regions of high interspecies variability near Arg 89 (see Fig. 9A shows the basic model for rat Thy-1 embedded in a
Table 1 and Fig. 9), and therefore presumably determine the lipid monolayer (for simplicity, the three N-linked carbohy-
spatially overlapping epitopes recognised by the rat anti-mouse drate chains have been omitted from the figure, but they were
Thy-1 antibodies (Pont et al. 1985). The CD spectrum of included in the calculations). The four Cys residues that form
human Thy-1 is quite distinct from that of rat Thy-1, being the two disulphide bonds are shown in yellow, the interspecies
apparently dominated by contributions from Tyr residues. variable regions (Table 1) are coloured green, blue and orange.
There appears to be no more than a small disuphide contribu- Three amino acid side chains are shown - that of Arg 89 (deter-
tion in human Thy-1, although this is hard to assess given the mining the Thy-1.1 epitope) projecting from the orange portion
low protein concentration used for the measurement. Delipi- of the ribbon, and of His 32 (from green section) and Arg 66
dation caused a marked shift in the CD spectrum of human (from the blue section), both of which in human Thy-1 are Tyr
Thy-1 (Fig. 8, traces c (before PI-PLC addition) and d (after residues. The glycan chain of the lipid anchor is folded tightly494 E. Barboni and others
Table 1. Regions of main sequence diversity between rat, mouse and human Thy-1 (Williams, 1989)
Residue no.: 25...........................32 60...............................68 84.......................90
Colour code: Green Blue Orange
Rat Thr Asn Leu Pro --- Ile Gln His Val Asn Leu Phe Ser Asp Arg Phe Ile Tyr Met Cys Glu Leu Arg Val
Mouse Thr Lys Asp Asn Ser Ile Gln His Val Thr Leu Ser Asn Gln Pro Tyr Ile Tyr Phe Cys Glu Leu Gln Val
Human Ser Ser Ser Pro --- Ile Gln Tyr Thr Asn Phe Thr Ser Lys Tyr Asn Met Tyr Thr Cys Ala Leu His His
Gln at residue 89 in mice is the allelic residue that is Arg for Thy-1.1; the two Tyr residues are found in human but not rat Thy-1.
20
which in human is a Tyr, changes its orientation markedly
0 (compare Fig. 9A,B), moving from 2.25 to 4.19 Å distant from
Arg 89 (i.e. outside van der Waals’ interaction distance),
-20 d whereas Leu 27 moves inside this distance (from 3.20 to
2.11Å). Arg 66 (Tyr in human) in the blue variable region on
Delta A (x106)
-40 c delipidation forms a H bond from its amide NH to the C=O of
Ser 64. These changes would account for the observed shift in
-60
the CD spectrum of human Thy-1. On the other hand, the two
-80
disulphide bonds, and the rat Tyr residues at positions 56 and
84, have virtually identical neighbouring residues within the
-100 2.5 Å van der Waals’ radius with both Thy-1 forms; their H-
a,b
bonding relationships are also preserved, although the OH of
-120 Tyr 56, which forms a H bond with the OH of Tyr 84, forms
an additional H bond on delipidation with the OH of Thr 48.
-140
250 270 290 310 330 350
This would account for the lack of effect of delipidation on the
CD spectrum of rat Thy-1, and exemplifies the general lack of
Wavelength (nm) conformational effect of delipidation on the Thy-1 protein
Fig. 8. Circular dichroism spectrum of rat (a,b; 0.1 mg/ml) and
except at the interspecies variable regions.
human (c,d; 0.01 mg/ml) Thy-1 before (a,c) and 1 hour after (b,d) To assess the extent to which the conformational shift seen
adding PI-PLC. Single traces are shown; in fact the spectra were in the model was due positively to binding of the delipidated
followed and found to be stable for 3 hours before adding the glycan chain to Thy-1, rather than to relaxation of a confor-
enzyme, and for 24 hours following delipidation. Delta A is the mation imposed by the lipid-anchored glycan, the entire glycan
differential absorbance (∆A=AL−AL), where AL and AL are the of the GPI anchor was removed and the model again subjected
absorbances for left- and right-handed circularly polarised light. to molecular dynamics. After 10 picoseconds, the protein con-
on itself, bringing its Manα(1-2)Man α(1-2)Man region close formation had relaxed somewhat, but not returned to that of
to the protein and GlcNH2-Ins close to the lipid bilayer. native Thy-1 (Fig. 9C). Thus Arg 89 broke its (delipidated) H
The phospholipid portion of the anchor was then removed, bond with Phe 34, but did not re-form the H bond with Ser 91
mimicking a PLD reaction, and the model subjected to 100 present in the model of native Thy-1; residues Leu 49, Val 50
picoseconds of dynamics. A new stable conformation was and Glu 52 moved back closer to Arg 89 (8.62, 9.80 and 14.04
reached within 10 picoseconds and is shown in Fig. 9B. The Å, respectively), but not to within the 7-8 Å of normal Thy-1.
delipidated glycan chain changed conformation, bringing its A similar effect was found elsewhere - Thy-1 with this entire
distal GlcNH2-Ins residues within less than 10Å of Val 11, Asn glycan chain removed had a conformation intermediate
12, Asp 18 and Leu 108. This produced a conformational shift between native and delipidated Thy-1, but was closer to that
in the Thy-1 protein that was most marked on the external face of the delipidated form.
of Thy-1, on the opposite side of the molecule to the GPI
anchor. Thus in the model of Thy-1 attached to lipid, Arg 89
forms a H bond from its amide NH to the C=O of Ser 91; after DISCUSSION
delipidation, it forms a new H bond, from its C=O to the NH
of Phe 34. The environment of Arg 89 changed even more Delipidation leads to a marked decrease in Thy-1 immunore-
remarkably with respect to the residues of the C” sheet (imme- activity, as assessed by a variety of antibodies in many types
diately behind Arg 89 in the view shown). Leu 49, Val 50 and of assay. The effect, analysed in detail with OX7 antibodies
Glu 52, that are 7-8 Å from Arg 89 in native Thy-1, moved to whose epitope is specified by Arg rather than Gln at residue
a distance of 9.30, 10.45 and 16.49 Å, respectively following 89, is primarily on the dissociation rate, which for this antibody
delipidation. This conformational shift predicted by the model is increased nearly 10-fold.
in the region of Arg 89 would be expected to affect the affinity Several relatively trivial mechanisms could explain the
of antibody binding to the allelic epitope. effect of delipidation upon antibody binding, but we believe
The two other interspecies variable regions also altered their these are precluded by our data.
conformation to an extent that would be expected to influence (1) Could the lipid group be directly involved in the allelic
antibody binding. Asn 26 (within the blue loop), for instance, epitope on Thy-1? This is unlikely on spatial grounds, readily
on delipidation loses a H bond with His 21, as does Ile 30 with seen from the model (Fig. 9A) where the two regions are more
Glu 33, and Gln 31 forms a new H bond with Pro 28. His 32, than 30 Å apart, whereas protein epitopes cover an area of 750-The GPI anchor affects Thy-1 protein conformation 495
B
A
C
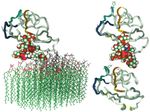
Fig. 9. Models of rat Thy-1 after molecular dynamics: (A) inserted into a lipid monolayer; (B) delipidated as though by PLD; and (C) with the
entire glycan chain of the GPI anchor removed. The protein is shown as a ribbon, the four Cys residues that form the two disulphide bonds are
in yellow (best seen in C), the interspecies variable regions (see Table 1) are coloured green (residues 25-32), blue (60-68) and orange (84-90).
The side chains of His 32 and Arg 66 (both Tyr in human), and Arg 89 (the residue determining the Thy-1.1 epitope), are shown. The glycan
portion of the GPI anchor is shown in space-filling CPK mode, the three N-linked carbohydrate chains have been omitted from the diagram for
simplicity.
900 Å2 (Davies et al., 1990). Moreover, all the antibodies used (3) Could the Thy-1, on removal of the hydrophobic anchor,
identify Thy-1 on the cell surface, where the lipid groups are change its physical form in solution (e.g. dimerise) and so
hidden in the membrane. We have recently shown that several exclude binding of antibody? This possibility is excluded on
of the antibodies used here (the anti-Thy-1.2 antibodies, and several grounds: such changes cannot be detected in gel filtra-
H140-150 and H154-177) react equally with native mouse tion or CD spectra, and are unlikely to be operative after immo-
Thy-1.2, and with hybrid molecules in which Thy-1 was bound bilisation of denatured Thy-1 on nitrocellulose in immunoblot-
to the membrane by a variety of transmembrane polypeptide ting. Moreover, such a mechanism would be expected
domains (Tiveron et al., 1994) and totally lacked a GPI anchor. primarily to affect the association of antibody with Thy-1,
It does not seem possible, therefore, that the GPI anchor could whereas the effect is primarily on dissociation.
be involved in these epitopes. (4) Could the Thy-1 preparation we use aggregate, allowing
(2) Could the phospholipid group, once removed by the higher affinity interaction with bivalent IgG antibodies
lipase, interact with some otherwise inaccessible site on Thy- (Karush, 1978), and this aggregation be dispersed upon delip-
1, and so perturb antibody binding? This seems highly unlikely idation to give the observed faster dissociation rate? We found
in detergent solution where the lipid is incorporated into monomeric Thy-1 isolated by gel filtration (whether in deoxy-
detergent micelles; and even more so where Thy-1 is removed cholate with its GPI anchor attached or delipidated in
from the cell surface by a lipase, leaving the lipid in the plasma detergent-free solution) did not aggregate even over prolonged
membrane. In immunoblots the lipid would be totally separated periods of storage (a year at 4°C), as assessed by its behaviour
from Thy-1 protein during SDS-PAGE, but the loss of on gel filtration (unpublished observations). In the experiments
antibody reactivity occurs. Such an interaction, therefore, can shown here, aggregation would produce multiphase kinetics,
be discounted. which should be particularly obvious in the dissociation496 E. Barboni and others
reaction (Morris, 1994), but was not observed. Disaggregation Bordier, C. (1981). Phase separation of integral membrane proteins in Triton
upon lipase treatment would have been evident in the CD X-114 solution. J. Biol. Chem. 256, 1604-1607.
spectrum of rat Thy-1, and was not seen (and light scattering, Brown, D. (1993). The tyrosine kinase connection: how GPI-anchored proteins
activate T cells. Curr. Opin. Immunol. 5, 349-354.
diagnositic of aggregation, was negligible). A disaggregation Buckle, P. E., Davis, R. J., Kinning, T., Yeung, D., Edwards, P. R., Pollard-
mechanism triggered by lipase action would have been Knight, D. and Lowe, C. R. (1993). The resonant mirror: a novel optical
detected by these experiments, and was not, confirming the biosensor for direct sensing of biomolecular interactions. Part II:
direct evidence that the Thy-1 in solution assays was Applications. Biosensors Bioelectronics 8, 355-368.
Campbell, D. G., Gagnon, J., Reid, K. B. M. and Williams, A. F. (1981). Rat
monomeric. brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an
(5) Proteolytic destruction of the epitopes is also incompat- unusual hydrophobic region. Biochem. J. 195, 15-30.
ible with the evidence: none is seen by SDS-PAGE, and the Cush, R., Cronin, J. M., Stewart, W. J., Maule, C. H., Molloy, J. and
existence of a small fraction of Thy-1 that resists extensive PI- Goddard, N. J. (1993). The resonant mirror: a novel optical biosensor for
PLC treatment, while retaining its reactivity with OX7 (the direct sensing of biomolecular interactions. Part I: Principles of operation
and asociated instrumentation. Biosensors Bioelectronics 8, 347-353.
fraction of Thy-1 migrating in the void volume in Fig. 3c,d), Davies, D. R., Padlan, E. A. and Sheriff, S. (1990). Antibody-antigen
is difficult to reconcile with a proteolytic mechanism. In fact, complexes. Annu. Rev. Biochem. 59, 439-73.
Thy-1 is very resistant to proteolysis, a property that proved a Durbin, H., Young, S., Stewart, L. M., Wrba, F., Rowan, A. J., Snary, D.
major problem in sequencing the protein (Campbell et al., and Bodmer, W. F. (1994). An epitope on carcinoembryonic antigen
defined by the clinically relevant antibody PR1A3. Proc. Nat. Acad. Sci. USA
1981). 91, 4313-4317.
The conclusion, therefore, appears inescapable: that delipi- George, A. J. T., French, R. R. and Glennie, M. J. (1995). Measurement of
dation triggers some major change in Thy-1 that substantially kinetic binding constants of a panel of anti-saporin antibodies using a
alters the region of the protein specifying the epitopes for these resonant mirror biosensor. J. Immunol. Meth. (in press).
antibodies. As the model makes clear, this implies a confor- Gooderham, K. (1984). High-sensitivity silver staining of proteins following
polyacrylamide gel electrophoresis. In Methods in Molecular Biology (ed. J.
mational shift on the face of Thy-1 furthest away from the GPI M. Walker), pp. 113-8. Clifton, NJ: Humana Press.
anchor, a shift that is confirmed by the CD spectral changes Greenspan, R. J. and O’Brien, M. C. (1989). Genetic evidence for the role of
and by molecular dynamics. This work therefore demonstrates Thy-1 in neurite outgrowth in the mouse. J. Neurogenet. 5, 25-36.
that the GPI anchor, when attached to the membrane (or a Homans, S. W., Ferguson, M. A., Dwek, R. A., Rademacher, T. W., Anand,
R. and Williams, A. F. (1988). Complete structure of the glycosyl
detergent micelle), imposes a conformation on the external phosphatidylinositol membrane anchor of rat brain Thy-1 glycoprotein.
face of Thy-1. The molecular dynamics further suggests that Nature 333, 269-72.
the major factor in this is the conformation of the glycan chain Hooper, N. M. (1992). More than just a membrane anchor. Curr. Biol. 2, 617-
imposed by its environment between the lipid surface and the 619.
protein. This clearly could operate for other GPI-linked Karush, F. (1978). The affinity of antibody: range, variability and the role of
multivalence. In Immunoglobulins (ed. G. W. Litman and R. A. Good), pp.
proteins, and appears to happen with the carcinoembryonic 85-116. New York: Plenum Medical Book Co.
antigen. An antibody binds the carboxy-terminal Ig domain of Kuchel, P. W., Campbell, D. G., Barclay, A. N. and Willliams, A. F. (1978).
this antigen only when it is anchored to the membrane, not Molecular weights of the Thy-1 glycoproteins from rat thymus and brain in
when it is released through endogenous lipase action (Durbin the presence and absence of deoxycholate. Biochem. J. 169, 411-417.
Laemmli, U. (1970). Cleavage of structural proteins during the assembly of
et al., 1994); a result that suggests that delipidation affects the bacteriophage T4. Nature 227, 680-681.
conformation of this glycan/Ig domain complex. The possibil- Ledbetter, J. A. and Herzenberg, L. A. (1979). Xenogeneic monoclonal
ity must therefore be considered that the GPI anchor generally antibodies to mouse lymphoid differentiation antigens. Immunol. Rev. 47,
affects the conformation of the protein domain to which it is 63-90.
attached. This clearly has broad functional implications for this Lisanti, M. P., Scherer, P. E., Tang, Z. and Sargiacomo, M. (1994).
Caveolae, caveolin and caveolin-rich membrane domains: a signalling
family of membrane proteins and, for instance, could well lie hypothesis. Trends Cell Biol. 4, 231-5.
behind the greater effectiveness of GPI-anchored, compared to Mason, D. W. and Williams, A. F. (1980). The kinetics and binding to
transmembrane, LFA-3 as a ligand for CD2 (Tozeren et al., membrane antigens in solution and at the cell surface. Biochem. J. 187, 1-20.
1992). Matthew, W. D., Greenspan, R. J., Lander, A. D. and Reichardt, L. F.
(1985). Immunopurification and characterization of a neuronal heparan
sulfate proteoglycan. J. Neurosci. 5, 1842-1850.
We thank Drs Hai-Tao He and Michel Pierres for the gifts of
Mayor, S., Rothberg, K. G. and Maxfield, F. R. (1994). Sequestration of GPI-
hybridoma cells; John Aherne and Deborah Wenham from Fisons anchored proteins in caveolae triggered by cross-linking. Science 264, 1948-
Applied Sensor Technology for their constant advice on the use of the 51.
IAsys and its associated software; Dr Mark Wormald and Professor Miyata, T., Isobe, K., Dawson, R., Ritter, M. A., Inagi, R., Oda, O.,
Raymond Dwek for the coordinates of the Thy-1 model; and Dr Willie Taguchi, R., Ikezawa, H., Inoue, I., Seo, H., Hasegawa, M., Kobayashi,
Taylor for assistance. This work was supported by the American S., Maeda, K., Yamada, K. and Nakashima, I. (1990). Determination of
Paralysis Association, the Muriel Edith Rickman Trust and the MRC. the molecular nature and cellular localization of Thy-1 in human renal tissue.
Immunology 69, 391-5.
Morris, R. J. and Raisman, G. (1983). Estimation of Thy-1 in cryostat
sections of nervous tissue. J. Neurochem. 40, 637-644.
REFERENCES Morris, R. J. (1994). Antigen-antibody interactions: how affinity and kinetics
affect assay design and selection procedures. In Monoclonal Antibodies (ed.
Almqvist, P. and Carlsson, S. R. (1988). Characterization of a hydrophilic M. A. Ritter and H. Ladyman), pp. 34-59. Cambridge, U.K.: Cambridge
form of Thy-1 purified from human cerebrospinal fluid. J. Biol. Chem. 263, University Press.
12709-15. Parekh, R. B., Tse, A. G., Dwek, R. A., Williams, A. F. and Rademacher, T.
Barboni, E., Gormley, A. M., Pliego Rivero, F. B., Vidal, M. and Morris, R. W. (1987). Tissue-specific N-glycosylation, site-specific oligosaccharide
J. (1991). Activation of T lymphocytes by cross-linking of patterns and lentil lectin recognition of rat Thy-1. EMBO J. 6, 1233-44.
glycophospholipid-anchored Thy-1 mobilizes separate pools of intracellular Perkins, S. J., Williams, A. F., Rademacher, T. W. and Dwek, R. A. (1988).
second messengers to those induced by the antigen-receptor/CD3 complex. The Thy-1 glycoprotein: a three-dimensional model. Trends Biochem. Sci.
Immunology 72, 457-63. 13, 302-3.The GPI anchor affects Thy-1 protein conformation 497 Pont, S., Regnier-Vigouroux, A., Naquet, P., Blanc, D., Pierres, A., Tozeren, A., Sung, K., Sung, L. A., Dustin, M. L., Chan, P. Y., Springer, T. Marchetto, S. and Pierres, M. (1985). Analysis of the Thy-1 pathway of T A. and Chien, S. (1992). Micromanipulation of adhesion of a Jurkat cell to a cell hybridoma activation using 17 rat monoclonal antibodies reactive with planar bilayer membrane containing lymphocyte function-associated antigen distinct Thy-1 epitopes. Eur. J. Immunol. 15, 1222-1228. 3 molecules. J. Cell Biol. 116, 997-1006. Rademacher, T. W., Edge, C. J. and Dwek, R. A. (1991). Dropping anchor Williams, A. F. and Gagnon, J. (1982). Neuronal cell Thy-1 glycoprotein: with the lipophosphoglycans. Curr. Biol. 1, 41-42. homology with immunoglobulin. Science 216, 696-703. Renouf, D. V. and Hounsell, E. F. (1993). Conformational studies of the Williams, A. F. (1989). The structure of Thy-1 antigen. In Cell Surface Antigen backbone (poly-N-acetyllactosamine) and the core region sequences of O- Thy-1. Immunology, Neurology and Therapeutic Applications (ed. A. E. Reif linked carbohydrate chains. Int. J. Biol. Macromol. 15, 37-42. and M. Schlesinger), pp. 49-70. New York and Basel: Marcel Dekker, Inc. Strickland, E. H. (1974). Aromatic contributions to circular dichroism spectra Williams, A. F., Parekh, R. B., Wing, D. R., Willis, A. C., Barclay, A. N., of proteins. CRC Crit. Rev. Biochem. 2, 113-175. Dalchau, R., Fabre, J. W., Dwek, R. A. and Rademacher, T. W. (1993). Tiveron, M. C., Nosten-Bertrand, M., Jani, H., Garnett, D., Hirst, E. M. A., Comparative analysis of the N-glycans of rat, mouse and human Thy-1. Site- Grosveld, F. and Morris, R. J. (1994). The mode of anchorage to the cell specific oligosaccharide patterns of neural Thy-1, a member of the surface determines both the function and membrane location of Thy-1 immunoglobulin superfamily. Glycobiology 3, 339-48. glycoprotein. J. Cell Sci. 107, 1783-96. Toutant, J. P., Roberts, W. L., Murray, N. R. and Rosenberry, T. L. (1989). Conversion of human erythrocyte acetylcholinesterase from an amphiphilic to a hydrophilic form by phosphatidylinositol-specific phospholipase C and serum phospholipase D. Eur. J. Biochem. 180, 503-8. (Received 18 July 1994 - Accepted 6 October 1994)
You can also read